Figure 7. Relative positioning of Gln118 and Asp170 at the AnsA active site.
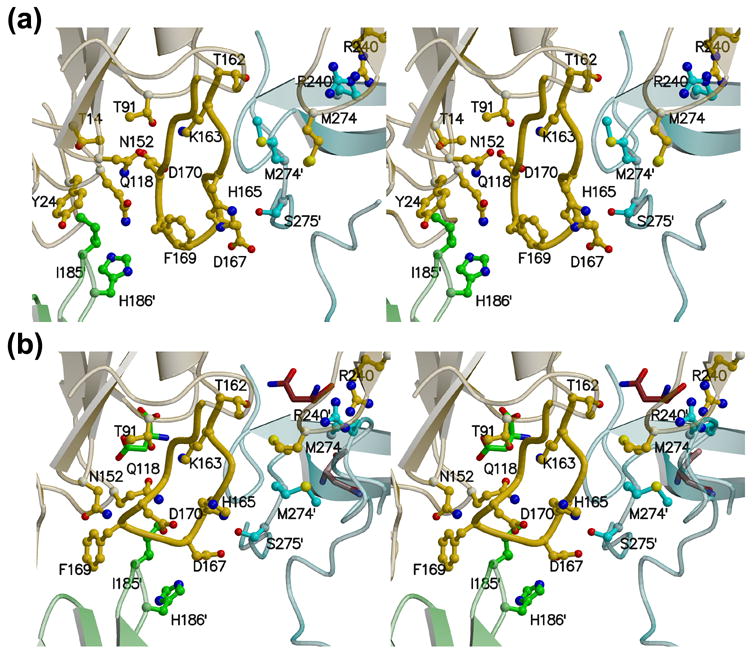
During the allosteric switch upon binding asparagine, the two loops containing Gln118 and Asp170 adjacent to the active site change conformation. As a result, Gln118 occupies the space adjacent to Thr91 and Lys163 that was previously occupied by Asp170 in the apo structure. (a) Stereoview of the apo active site. (b) Stereoview of the AnsA-asparagine active site. In each panel, monomer A is yellow, B is green and C is cyan. Note how the active site locale is at the convergence of three subunits. Details are provided in the text.
