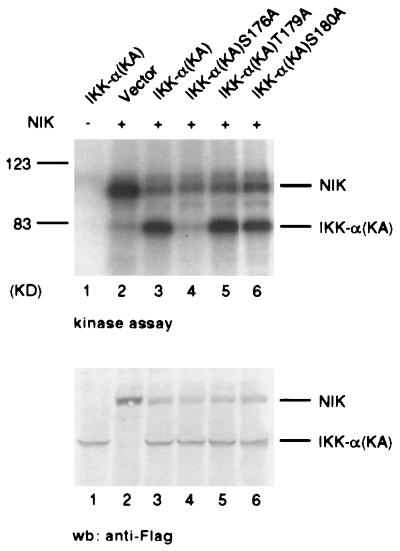
Figure 3.
Ser-176 in the activation loop of IKK-α is a major site of phosphorylation by NIK. Individual serine and threonine residues in the activation loop of IKK-α kinase domain were mutated to alanine. Each IKK-α mutant protein also contained the KA mutation in the ATP-binding site to prevent autophosphorylation. 293 cells were transiently transfected with expression plasmids encoding the indicated FLAG epitope-tagged proteins. Thirty-six hours after transfection, immunopurified proteins were incubated with [γ-32P]ATP, resolved by SDS/PAGE, and analyzed by autoradiography. The amount of protein used in each reaction was determined by immunoblotting (wb) with anti-FLAG polyclonal antibodies (Lower).