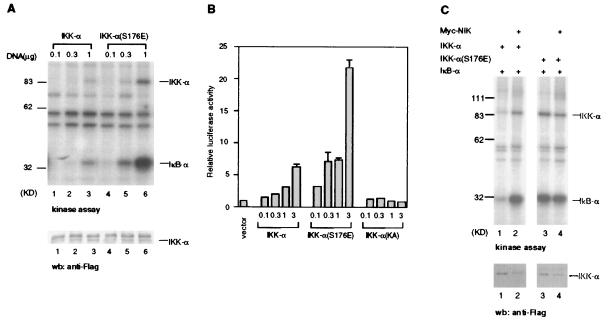
Figure 7.
IKK-α(S176E) is constitutively active. (A) IKK-α(S176E) has significantly greater activity than IKK-α in kinase assay. HeLa cells were transiently transfected with expression plasmids for FLAG epitope-tagged IKK-α or IKK-α(S176E) at different doses. Thirty hours later, IKK-α proteins were purified with anti-FLAG antibodies, and in vitro phosphorylation reactions were carried out with bacterially expressed IκB-α and [γ-32P]ATP. The amounts of protein used were determined by immunoblotting with anti-FLAG antibodies (as shown in the lower panel). (B) IKK-α(S176E) has significantly greater activity than IKK-α in an NF-κB reporter gene assay. HeLa cells were transiently cotransfected with an E-selectin-luciferase reporter gene plasmid and vector control or IKK-α expression vector as indicated. Twenty-four hours after transfection, luciferase activities were determined and normalized on the basis of β-gal expression. The values shown are averages (±SEM) of duplicate samples for one representative experiment. (C) IKK-α(S176E) activity is independent of NIK activation. HeLa cells were transiently transfected with expression plasmids for FLAG epitope-tagged IKK-α or IKK-α(S176E) and Myc-epitope-tagged NIK. IKK-α proteins were purified with anti-FLAG antibodies, and in vitro phosphorylation reactions were carried out by using bacterially expressed IκB-α and [γ-32P]ATP. The amounts of IKK-α protein used were determined by immunoblotting with anti-FLAG antibodies (Lower).