Fig. 5. Reduction of hemes bn/cn by reduced ferredoxin/FNR.
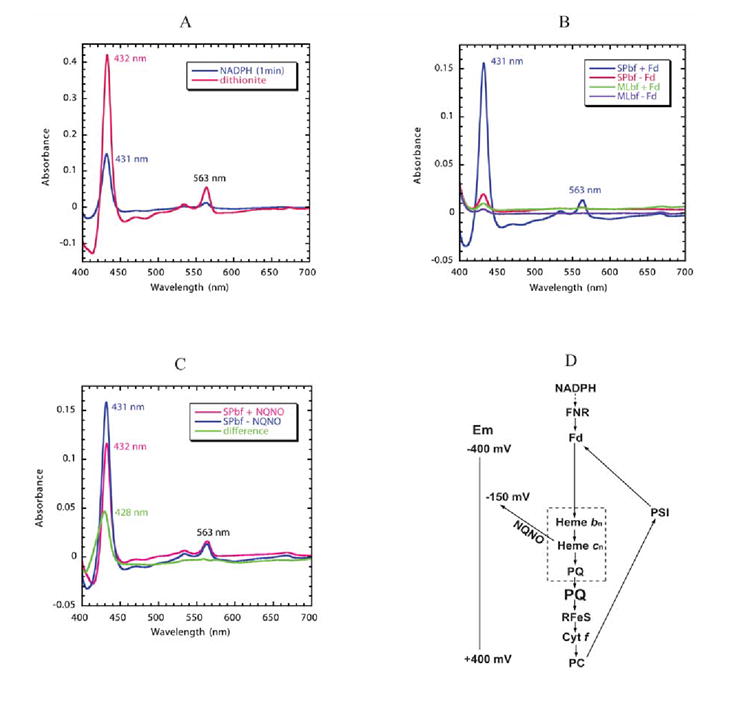
The reaction solution contained 1.5 μM spinach b6f complex with bound FNR and 3 μM spinach ferredoxin. Semi-anaerobic conditions were obtained through the addition of 10 mM glucose and 160 units of glucose oxidase. Decyl-plastoquinol (50 μM) was present to eliminate any contribution of cytochrome f to the difference spectra. Cytochrome b6 /cn reduction was initiated by addition of 0.3 mM NADPH. (A) Comparison of reduction of spinach b6f complex by NADPH/ferredoxin 14 and dithionite. The NADPH/ferredoxin minus decyl-plastoquinol difference spectrum (blue trace) was measured 1 min after addition of NADPH. The spectrum of fully reduced cytochrome b6 (pink) was obtained by addition of dithionite. (B) Dependence of reduction of hemes bn/cn reduction on the presence of FNR and ferredoxin. Reduction of spinach b6f complex following addition of NADPH in the presence (blue) or absence (red) of ferredoxin (3 μM), and of M. laminosus b6f complex, which does not have bound FNR, in the presence (green) or absence (purple) of ferredoxin. All spectra were measured 1 min after addition of NADPH. (C) Double difference spectrum, normalized at 563 nm, showing the Soret band spectrum of heme cn (green), measured as the difference between the spectrum obtained in the presence and absence of NQNO. Difference spectra obtained in the absence (blue) and presence (pink) of NQNO (final concentration, 25 μM). (D) Proposed pathway of reduction of hemes bn and cn by reduced ferredoxin (Fd) in a Q cycle or cyclic pathway in which ferredoxin is reduced physiologically by the photosystem I reaction center and by NADPH in the present experiment. The requirement of FNR and Fd for the pathway of heme bn reduction by NADPH is defined by the data shown in Fig. 5B. The ability of NQNO to block the pathway at the site of heme cn is ascribed to NQNO binding as a ligand of this heme (Fig. 4B) and the NQNO-induced decrease in Em7 of heme cn 55. The inference that hemes bn and cn, together with PQ, are part of a complex in which PQ serves as a ligand of heme cn is based on the proximity of the two hemes 8; 9, their interaction measured by EPR 66, and binding of the quinone analogue inhibitors, TDS (Fig. 3B) and NQNO (Fig. 4B).
