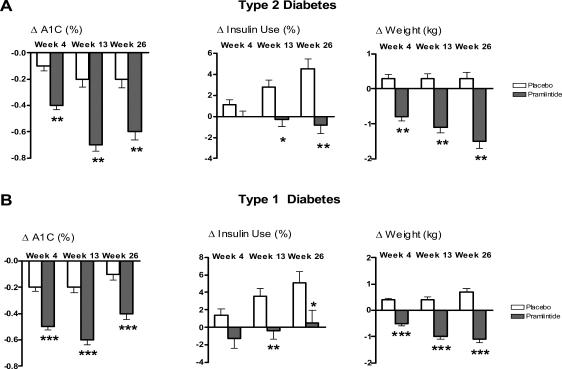
Figure 5.
Results for combined pivotal studies with pramlintide in patients with type 1 and type 2 diabetes. The change in A1C, total daily insulin use and weight at 4, 13, and 26 weeks are expressed as mean ± SE, ITT observed. (A) Patients with type 2 diabetes at 120 μg pramlintide BID (n=292) versus placebo (n=284), *p<0.01, **p<0.0001. (B) Patients with type 1 diabetes, 30–60μg doses TID and QID (n=716) versus placebo (n=538), *p<0.05, **p<0.01, ***p<0.0001 (combined data adapted from Ratner et al 2002, 2004; Whitehouse et al 2002; Hollander et al 2003).
Abbreviations: A1C, glycosylated hemoglobin; ITT, intention to treat; QID, four times daily; SE, standard error of mean; TID, three times daily.