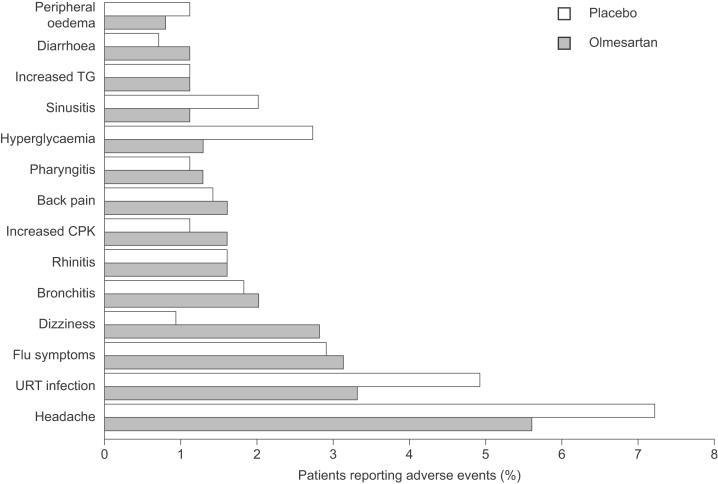
Figure 3.
Adverse events reported by >1% of patients with hypertension in a meta-analysis of seven randomized clinical trials (Neutel 2001) in which patients with hypertension randomly were randomized to olmesartan medoxomil (various doses from 2.5mg/day to 80mg/day; n=2540) or placebo (n=555) for 6 to 12 weeks. Copyright © 2002. Reproduced with permission from Warner GT, Jarvis B. 2002. Olmesartan medoxomil. Drugs, 62:1345-53; discussion, 1354-6.
Abbreviations: CPK, creatine phosphokinase;TG, triglyceride; URT, upper respiratory tract.