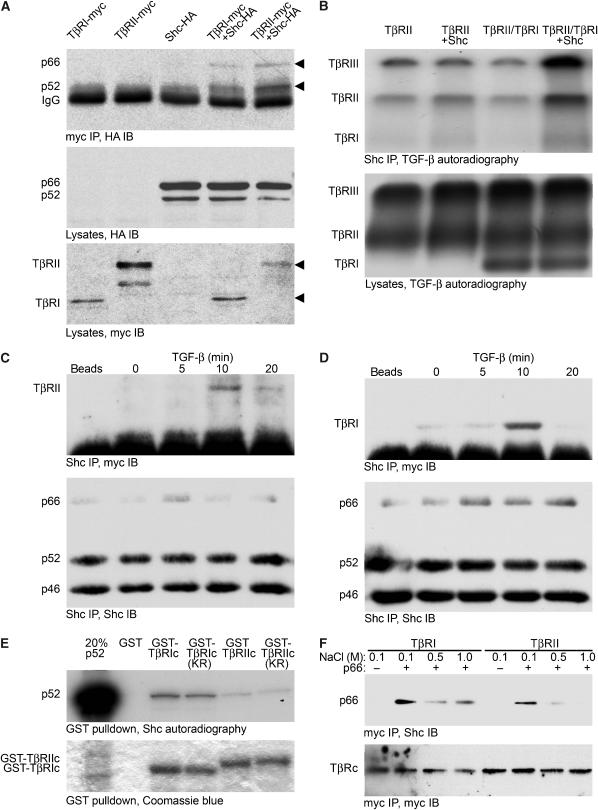
Figure 4.
ShcA interacts with TGF-β receptors. (A) p66ShcA and p52ShcA co-precipitate with TGF-β receptors in COS cells. Cells expressing myc-tagged TβRI or TβRII, and/or HA-tagged p66ShcA and p52ShcA, as indicated, were subjected to myc immunoprecipitation followed by HA immunoblotting (top). Expression of ShcA and TGF-β receptors was monitored by HA (middle) or myc (bottom) Western analysis of cell lysates. (B) ShcA interacts with cell surface TGF-β receptors. 3T3-Swiss cells were transfected to express TβRII and/or TβRI with or without p66ShcA, and cell surface receptors were radiolabelled with 125I-TGF-β1 followed by chemical crosslinking. Immunoprecipitation of both endogenous and transfected ShcA followed by autoradiography (top panel) demonstrated co-precipitation of 125I-labelled TGF-β receptors with ShcA. 125I-labelled TGF-β receptors did not precipitate when the ShcA antibody was replaced with non-immune IgG (data not shown). Autoradiography of total cell lysates (below) showed equivalent levels of 125I-labelled TGF-β receptors. (C, D) Ligand-dependent interaction of endogenous ShcA and TGF-β receptors in mammalian cells. 3T3-Swiss cells were treated with 4 ng/ml TGF-β for the indicated times, lysed, and subjected to ShcA immunoprecipitation followed by TβRII (C) or TβRI (D) immunoblotting. Control precipitations without primary antibody are shown (‘beads'). Subsequent ShcA immunoblots (lower panels) confirmed equivalent precipitation efficiencies. (E) In vitro association of p52ShcA with TβRI and TβRII cytoplasmic domains. In vitro translated 35S-labelled p52ShcA was incubated with GST, GST-fused TβRI or TβRII cytoplasmic domains, or their kinase-deficient point mutants (TβRIc, TβRIIc, TβRIc KR, and TβRIIc KR, respectively). 35S-labelled ShcA adsorbed to the GST fusion proteins was visualised by SDS–PAGE and autoradiography (upper panel). To confirm identity, 35S-labelled p52ShcA was loaded in lane 1 at 20% of the volume used in the binding assay. Equivalent GST fusion protein loading was confirmed by Coomassie blue staining. (F) ShcA association with TβRI and TβRII cytoplasmic domains in vitro depends on NaCl concentration. Myc-tagged proteins incorporating either TβRIc or TβRIIc were expressed in COS cells, isolated by myc immunoprecipitation, and bound to recombinant p66ShcA. The complexes were washed in otherwise identical buffers containing the indicated NaCl concentrations and subjected to ShcA immunoblotting (above). Myc Western analysis of the blot confirmed equivalent recovery of TβRIc and TβRIIc (below).