Figure 2. Immobilized metal affinity chromatography enables purification of A2aR from batch culture using two lysis methods.
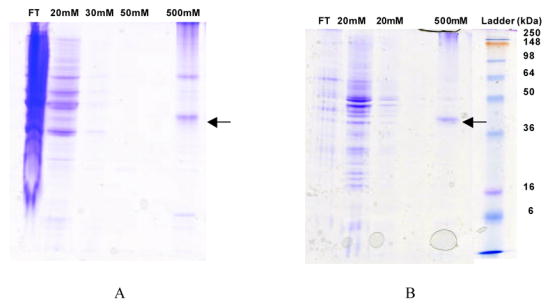
Coomassie-stained 12% SDS-PAGE gels illustrate the purification process. (A) A2aR-His10 was eluted using 500 mM imidazole as recovered using the bead-vortexing lysis method. (B) A2aR-His10 was also purified using the membrane solubilization method. Similar results were obtained for the purification using both methods. All samples were solubilized in purification buffer containing 2% DDM, 1% CHAPS, and 0.2% CHS and supplemented with protease inhibitors and 1 mM PMSF. Wash steps were carried out in purification buffer containing 0.1% DDM, 0.1% CHAPS, and 0.02% CHS supplemented with protease inhibitors and 1 mM PMSF. This cell/surfactant flow-through (FT) denotes proteins that were present in the crude lysate, and did not bind to the resin. 20 mM–50 mM lanes show proteins which were removed from the resin during washes with low-concentration imidazole. The ladder is See-BluePlus2 protein molecular weight standard, with molecular weights indicated. A2aR-His10 monomer is denoted with the arrows.
