Figure 1.
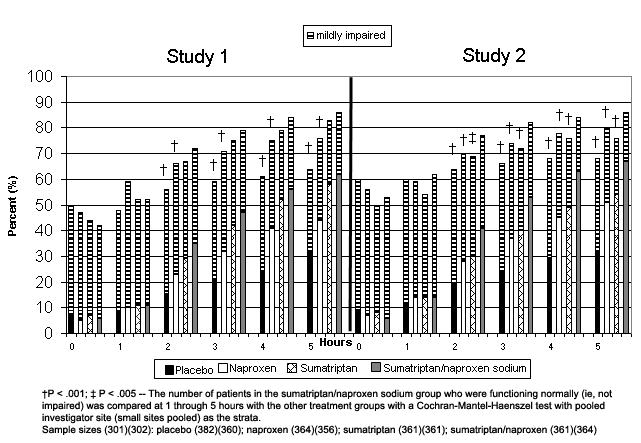
Proportion of patients reporting normal functioning or mild impairment from baseline to 5 hours post treatment.
† P < .001; ‡ P < .005 – The number of patients in the sumatriptan/naproxen sodium group who were functioning normally (ie, not impaired) was compared at 1 through 5 hours with the other treatment groups with a Cochran-Mantel-Haenszel test with pooled investigator site (small sites pooled) as the strata.
Sample sizes (301)(302): placebo (382)(360); naproxen (364)(356); sumatriptan (361)(361); sumatriptan/naproxen (361)(364)
