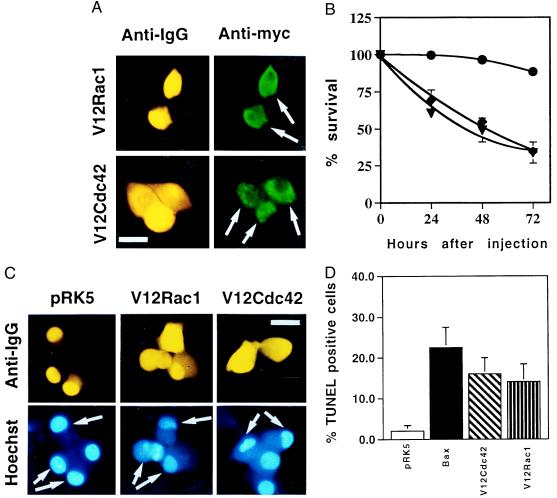
Figure 1.
Activated Cdc42 and Rac1 induce neuronal apoptosis. (A) The activated V12 mutants of Cdc42 and Rac1 were subcloned into the pRK5 mammalian expression vector and tagged with a myc epitope. Sympathetic neurons (SCG neurons), cultured for 5–7 days in the presence of NGF, were microinjected with 0.1 mg/ml DNA and 5 mg/ml guinea pig IgG, to follow the injected cells. For each experiment, 200 cells were microinjected. Four to 24 hr after injection, the cells were stained to check for expression of Rac1 and Cdc42 as described in Materials and Methods. (Bar = 100 μm.) The white arrows indicate the expressing cells. (B) Survival of SCG neurons injected with V12Cdc42 (triangles), V12Rac1 (squares), or pRK5 (circles). Twenty-four, 48, and 72 hr later, the percentage of surviving cells was assessed as described in Material and Methods. In each experiment, 200 cells were injected. The results are the means of three independent experiments ± SEM. (C) Morphology of the cells microinjected with V12Cdc42, V12Rac1, or pRK5. Six-day-old SCG neurons were injected with 0.3 mg/ml DNA together with guinea pig IgG and stained with Hoechst 24 hr after injection. White arrows indicate the injected cells: only the cells overexpressing the constitutively active forms of Rac1 and Cdc42 displayed clearly pyknotic nuclei. (Bar = 100 μm.) (D) TUNEL analysis of SCG neurons microinjected with V12Cdc42, V12Rac1, pRK5, or Bax. Six-day-old SCG neurons were microinjected with 0.3 mg/ml V12 Rac1, V12Cdc42, pRK5 DNA, or 0.05 mg/ml Bax DNA. TUNEL analysis was performed 16 hr later. The results are the means of three independent experiments ± SEM.