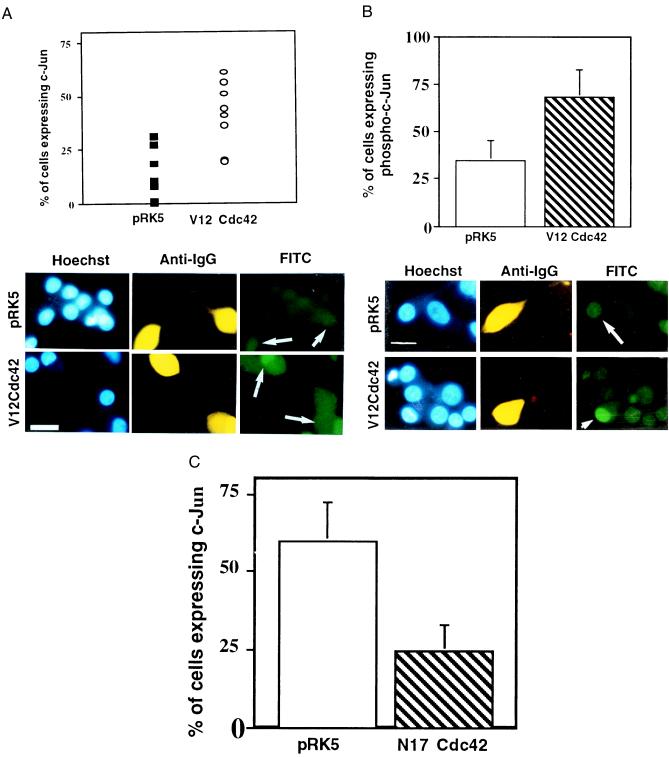
Figure 3.
Activation of Cdc42 results in an increase in the level of c-Jun protein and of its phosphorylation. (A) V12Cdc42 or pRK5 (0.3 mg/ml) was microinjected into 5- to 7-day-old SCG neurons, which were maintained in the presence of NGF. Twenty-four hours after injection, the cells were fixed, permeabilized, and stained with Hoechst dye (Left), a rhodamine-conjugated anti-guinea pig IgG antibody to detect the injected cells (Center), and an anti-c-Jun antibody (Right). Only the cells in which c-Jun staining was clearly above background were scored as positive. The results are presented as a scatter plot (Upper). The data are the means ± SEM of six independent experiments. Cdc42 induced a 2-fold increase in the percent of cells expressing c-Jun (white arrows) (P < 0.02). (Bar = 100 μm.) (B) V12Cdc42 or pRK5 (0.3 mg/ml) was microinjected into 5- to 7-day-old SCG neurons, which were kept in the presence of NGF. Twenty-four hours after injection, the percent of cells expressing phospho-c-Jun was assessed. Only the cells in which phospho-c-Jun staining was clearly above background were scored positive. The results are presented as a bar graph (Upper). The data are the means ± SEM of four independent experiments; P < 0.03. Cdc42 is capable of inducing a significant increase in the level of phospho-c-Jun in the injected cells (white arrows). (Bar = 100 μm.) (C) N17Cdc42 or pRK5 (0.6 mg/ml) was microinjected into 5- to 7-day-old SCG neurons, together with guinea pig IgG. The neurons were withdrawn from NGF 4–6 hr after injection. Twenty-four hours later, the percentage of cells expressing nuclear c-Jun was assessed. The results were represented as a bar graph. The data are the means ± SEM of six independent experiments. N17Cdc42 can block the induction of the increase in the level of c-Jun that is normally observed after NGF withdrawal (white arrows). (Bar = 100 μm.)