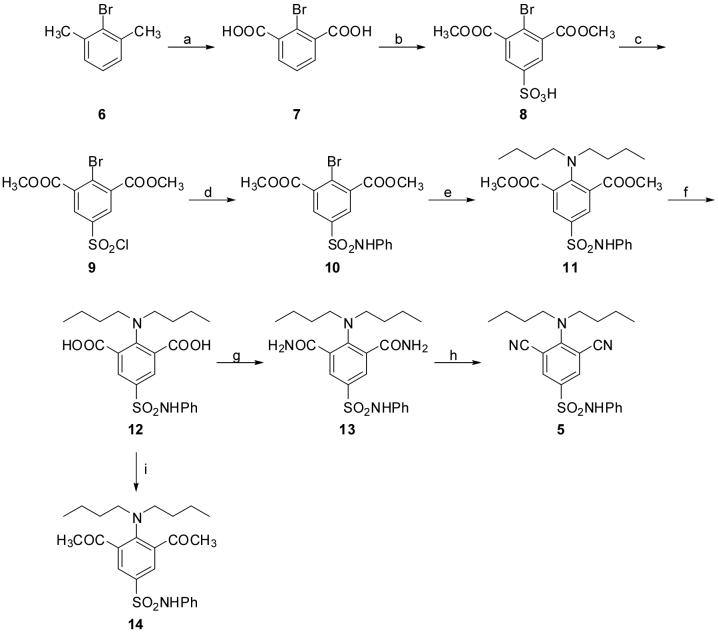
Scheme 2.
Reagents and conditions: (a) KMnO4, H2O, reflux, 66%; (b) (1) fuming H2SO4, 170 °C; (2) HCl, MeOH, reflux, 79% after two steps; (c) POCl3, reflux, 70%; (d) aniline, pyridine, acetone, 0 °C to rt, 66%; (e) dibutylamine, K2CO3, Cu, dioxane, reflux, 79%; (f) 3N NaOH, MeOH, reflux, 88%; (g) (1) CCl3COCCl3, PPh3, THF, 0 °C; (2) NH3/dioxane, rt, 68% after two steps; (h) PdCl2, CH3CN/H2O, 70 °C, 71%; (i) (1) CCl3COCCl3, PPh3, THF, 0 °C; (2) MeMgBr /THF, CuBr, THF, 0 °C, 55% after two steps.