Figure 1.
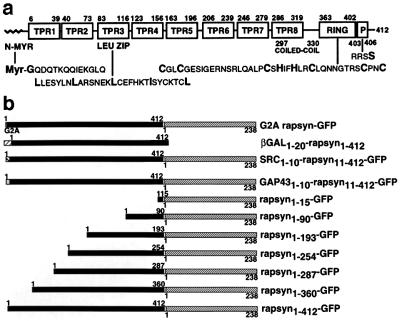
(a) Structural domains of rapsyn. The N terminus of rapsyn is myristoylated (N-Myr). Indicated are the N-terminal 10 amino acids absolutely conserved across species as well as rapsyn83–116, a leucine zipper motif (Leu zip), and rapsyn363–402, the cysteine rich (RING-H2) domain. Rapsyn403–406 is a consensus sequence for sites of both PKA and PKC phosphorylation. Also indicated are the borders of the eight putative TPRs and the borders of the predicted coiled-coil domain. (b) Chimeric proteins consisting of rapsyn, N-terminal modifications of full length rapsyn, or C-terminal deletions of rapsyn, each fused at its C terminus via an 8-amino acid linker with GFP, were constructed as described in Experimental Procedures.