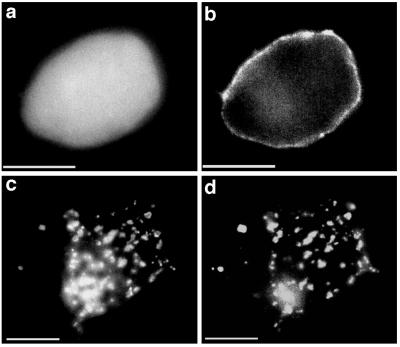
Figure 2.
Rapsyn-GFP, but not GFP, clusters nAChRs. cDNAs encoding nAChR subunits and GFP (a and b) or rapsyn-GFP (c and d) were transfected into HEK293T cells. Surface nAChRs in unfixed, nonpermeabilized cells were labeled sequentially with αBgTx, rabbit anti-αBgTx, and rhodamine-conjugated goat-anti-rabbit IgG. GFP or rapsyn-GFP was visualized with fluorescein isothiocyanate-optics (a and c) and nAChRs with rhodamine-optics (b and d). GFP, a cytosolic protein, was distributed throughout the cytoplasm (a) and did not interact with nAChRs distributed diffusely at the cell surface (b). Tagging of rapsyn with GFP did not interfere with the ability of rapsyn to form clusters (c) or to cluster nAChRs (d) that were colocalized with the rapsyn-GFP clusters (seen in all cells that expressed both proteins). (Bar = 10 μm.)