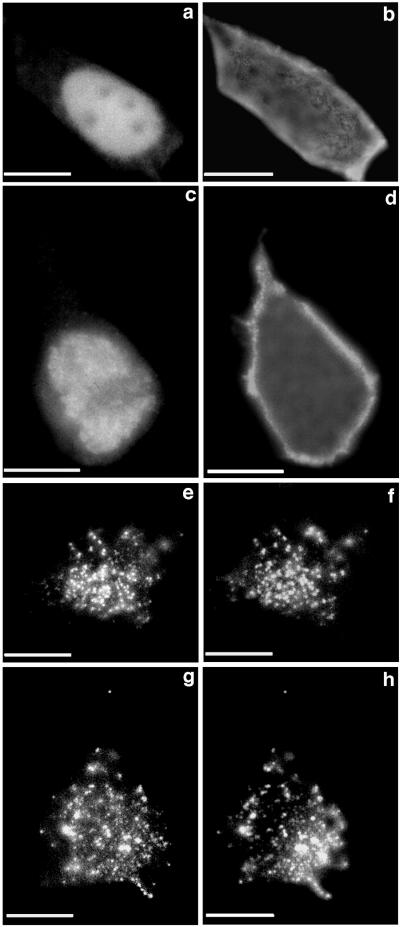
Figure 3.
N-terminal fatty acid modification of rapsyn is essential for targeting of rapsyn-GFP to the plasma membrane. HEK293T cells were cotransfected with cDNAs for nAChR subunits and N-terminal mutants of rapsyn: rapsynG2A-GFP (a and b); β-gal1–25-rapsyn (c and d); src1–10-rapsyn11–412-GFP (e and f); GAP431–10-rapsyn11–412-GFP (g and h). Distributions of rapsyn (Left) and nAChRs (Right) were visualized with fluorescein isothiocyanate and rhodamine optics, respectively. Mutation of Gly-2 to Ala (a and b) or addition of 25 amino acid residues of β-gal to the N terminus of rapsyn (c and d), which prevents N-myristoylation, resulted in targeting of mutant rapsyn-GFP or β-gal-rapsyn to the nucleus without affecting the surface expression of nAChRs. Substitution of the N-terminal 10 amino acids of rapsyn-GFP by that of the mouse src sequence (e and f), which also contains consensus sequence for N-myristoylation but a different amino acid sequence, or by that of GAP43 (g and h), which has a consensus sequence for S-palmitoylation, did not affect the plasma membrane targeting and self-association of mutant rapsyn-GFP or its ability to cluster nAChRs. (Bar = 10 μm.)