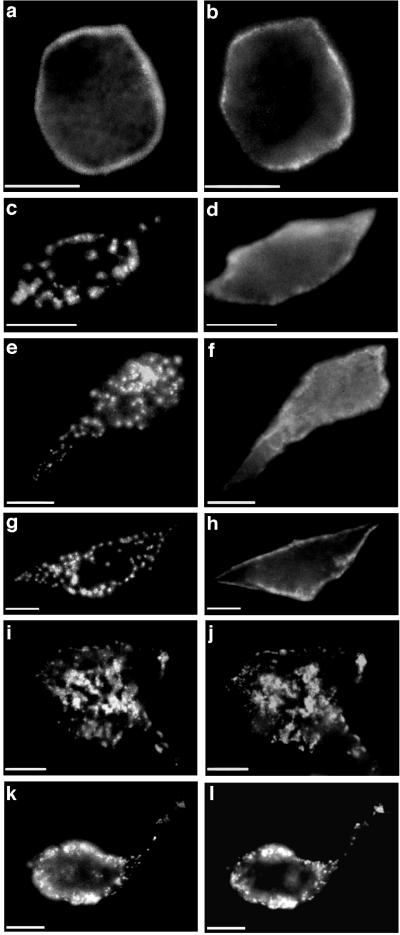
Figure 4.
Nonoverlapping structural domains within rapsyn are involved in self-association and nAChR clustering. The distributions of rapsyn (Left) and nAChRs (Right) were visualized in cells coexpressing nAChRs and N-terminal fragments of rapsyn tagged by GFP. (a and b) Rapsyn1–15-GFP was targeted to the plasma membrane but failed to self-associate or cluster nAChRs. (c and d) Rapsyn1–90-GFP was targeted to the plasma membrane and formed distinct clusters similar to wild-type rapsyn-GFP but did not cluster nAChRs. Rapsyn1–90-GFP was clustered in 82/100 cells positive both for GFP and nAChRs, while nAChRs were not clustered in any cells. (e and f) Rapsyn1–193-GFP and (g and h) rapsyn1–287-GFP also formed distinct clusters at the cell surface but failed to cluster nAChRs. Rapsyn1–287-GFP was clustered in 100/100 cells positive both for GFP and nAChRs, while nAChRs were distributed diffusely in 90/100 cells. nAChR clusters were colocalized with rapsyn1–287-GFP in 3/100 cells, while in 7/100 cells there was a granular distribution of nAChRs not colocalized with rapsyn1–287-GFP. (i–l) Rapsyn1–360-GFP formed distinct clusters at the cell surface (i, k, two representative cells) and formed nAChR clusters (j and l) similar in size to those formed by wild-type rapsyn-GFP. nAChRs were colocalized with rapsyn1–360-GFP clusters in 99/100 cells positive for both GFP and nAChR. In 1–2 cells there was a granular distribution of nAChRs not colocalized with rapsyn1–360-GFP. (Bar = 10 μm.)