Fig. 3.
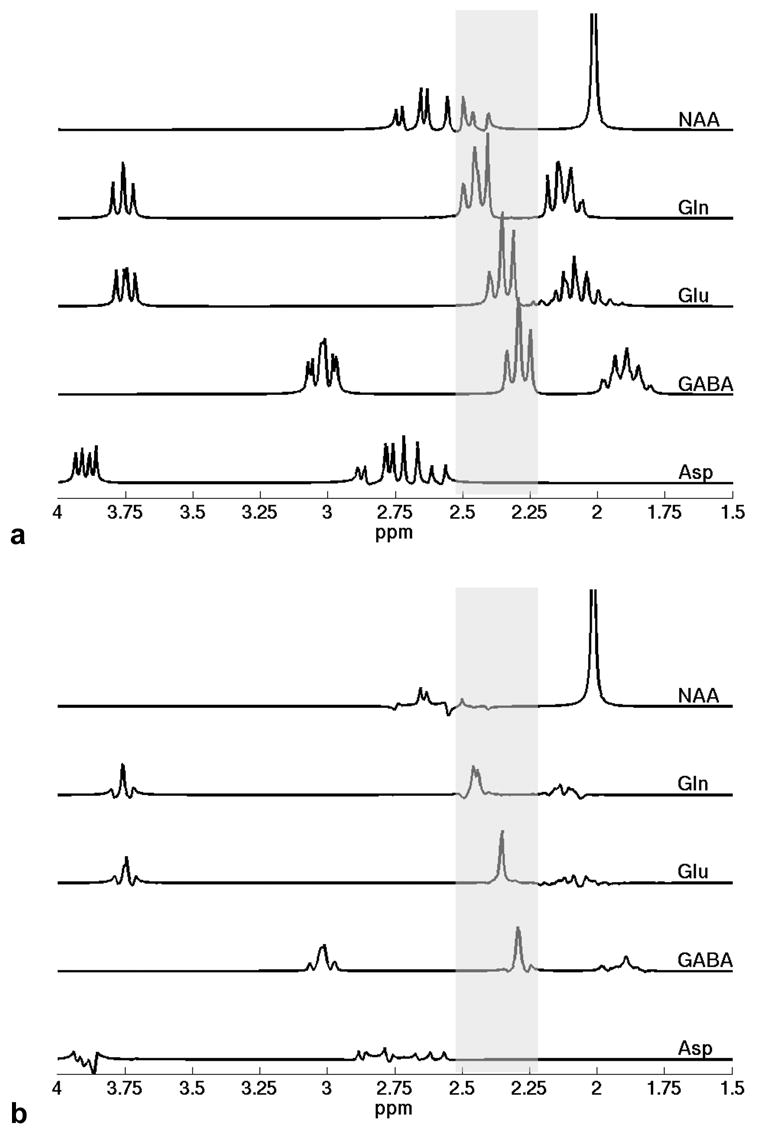
Simulated spectra of several possible contributing metabolites in the 2.2–2.5 ppm region, indicated by the shaded bars, at (a) typical short-echo parameters of TE = 10 ms/TM = 10 ms and (b) the optimized timing parameters of TE = 82 ms/TM = 48 ms, both under the same amplitude scale. The C3 multiplet resonance of the aspartyl group of NAA overlaps with the entire Gln C4 multiplet resonance from 2.37 to 2.51 ppm. Fortunately, the spectral contamination of NAA to Gln is rather low at the optimized sequence timing parameters (also shown in Fig. 2f). The spectral contamination of NAA to the Glu C4 multiplet resonance around 2.35 ppm and the GABA C2 multiplet resonance around 2.28 ppm is negligible, and the spectral resonances of Asp are completely outside the target region. The nonnegligible C3 multiplet resonances of Gln, Glu, and GABA overlap with the singlet resonance of the N-acetyl group of NAA at 2.02 ppm at short echo time in (a). In contrast, these C3 multiplet resonance signals decay significantly at the optimized sequence timing parameters in (b), thus greatly reducing the interference with quantification of NAA.
