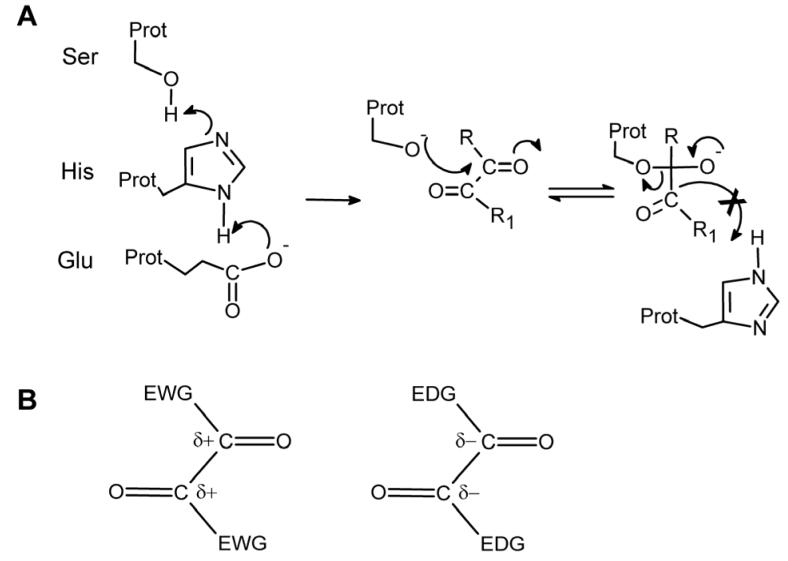
Figure 1.
(A) Proposed mechanism of interaction of the benzil analogues with the catalytic amino acids of CEs. A serine nucleophile is generated by proton transfer to a glutamic acid via a histidine residue, and the resulting oxygen atom attacks one of the carbonyl groups within the dione moiety. The tetrahedral intermediate that is generated is relatively stable, due to the increased strength of the C-C bond as compared to the C-O bond present in esters. Therefore the former bond is not cleaved resulting in inhibition of the enzyme. (B) Increasing or decreasing the electron density surrounding the carbonyl carbon atoms by introducing either electron withdrawing groups (EWG) or electron donating groups (EDG) within the molecules, should make the compounds better and poorer enzyme inhibitors, respectively.