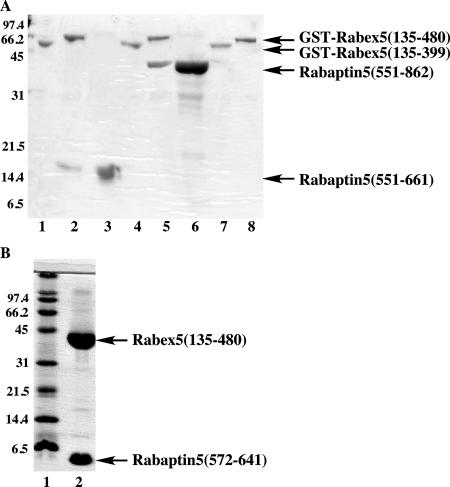
Figure 1.
Domains involved in Rabex-5 and Rabaptin-5 interaction. (A) The GST-Rabex-5 proteins (5 μg) were used to pull-down purified Rabaptin-5(551-661) (5 μg) in a 200-μl reaction. The bound proteins were analyzed by SDS-PAGE and visualized by Coomassie blue staining. Lane 1, GST-Rabex-5(135-399) incubation with 6-His-Rabaptin-5(551-661); lane 2, GST-Rabex-5(135-480) incubation with 6-His-Rabaptin-5(551-661); lane 3, 6-His-Rabaptin-5(551-661) directly loaded as a control; lane 4, GST-Rabex-5(135-399) incubation with 6-His-Rabaptin-5(551-862); lane 5, GST-Rabex-5(135-480) incubation with 6-His-Rabaptin-5(551-862); lane 6, 6-His-Rabaptin-5(551-862) directly loaded as a control; lane 7, GST-Rabex-5(135-399) directly loaded as a control; lane 8, GST-Rabex-5(135-480) directly loaded as a control. Molecular mass standards (in kilodaltons) are indicated on the left of the panel. (B) 6-His-tagged Rabex-5(135-480) was coexpressed with Rabaptin-5(572-641) in E. coli BL21 (DE3) and affinity purified with the His-Select resin (Sigma-Aldrich), followed by thrombin cleavage and further purification with Resource Q ion-exchange chromatography (GE Healthcare). Lane 1, molecular mass standards (in kilodaltons); lane 2, purified Rabex-5(135-480) and Rabaptin-5(572-641) complex.