Abstract
The role of spinal metabotropic glutamate receptors (mGluRs) in control of lower urinary tract functions was evaluated in rats using an mGluR antagonist administered via the intrathecal route. Cystometrograms in combination with external urethral sphincter (EUS) EMG recordings were performed on 13 decerebrate unanesthetized Sprague-Dawley female rats (n=6 for spinal cord intact rats; n=7 for spinal cord transected rats). In spinal cord intact rats, a group I/II mGluR antagonist, (+/−)-alpha-methyl-4-carboxyphenylglycine (MCPG), at doses of 3–30 μg changed neither bladder nor EUS EMG activity, whereas a larger dose (100 μg) produced a significant facilitation of EUS EMG activity (41% increase in the peak activity) with little effect on bladder contractions. In chronically spinal cord transected rats, MCPG (3–100 μg) had no effect on bladder and EUS EMG activity. The results suggest that group I/II mGluRs are likely to be involved in inhibition of the excitatory pathway to the EUS but not involved in the control of the bladder. The lack of effect of MCPG on the EUS EMG activity in chronic SCT rats indicates that mGluR-mediated inhibitory control of the EUS was eliminated after spinal cord injury.
Keywords: External urethral sphincter, Sphincter motor nucleus, Urinary bladder, Sacral parasympathetic nucleus, Micturition
Introduction
Glutamatergic receptors in the nervous system consist of two major classes, the ionotropic receptors which form ligand-gated cation channels [16] and metabotropic receptors (mGluRs) which are a family of G-protein coupled receptors activating distinct signal transduction pathways [4]. The former which include N-methyl-D-aspartate (NMDA) receptors and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors have an essential role in control of micturition [19,20,21]; however less is known about the function of mGluRs in micturition.
The mGluRs consist of eight subtypes (mGluRs1 to 8) that are divided into three groups (groups I to III) on the basis of sequence homology, transduction mechanisms, and response to various ligands [4]. Previous studies using trans-(+/−)-1-amino-1,3-cyclopentanedicarboxylic acid (ACPD), an agonist for groups I (mGluR1 and mGluR5) and II (mGluR2 and mGluR3) receptors, demonstrated that glutamatergic transmission via the mGluRs was involved in autonomic functions such as cardiovascular regulation [13,14] and respiration [15]. A recent study [18] demonstrated that mice lacking mGluR1 exhibited facilitated external urethral sphincter (EUS) EMG activity during voiding, raising the possibility that mGluR1 receptors have an inhibitory function in EUS reflex pathways. An immunohistochemical study also revealed mGluR1 immunoreactivity in the region of the EUS motor nucleus in the spinal cord [1,2]. Thus, the present study using an antagonist for groups I/II mGluRs was conducted to determine if these receptors participate in regulation of EUS activity and/or micturition in rats.
Some of the data have been reported previously in an abstract [22].
Materials and Methods
Thirteen female Sprague-Dawley rats (Charles River Laboratories, Boston, MA) weighing 235–283 g (mean=267 ± 4 g) were used in this study. The animals were housed under a 12 h light/dark cycle with controlled humidity and temperature. Standard pellet diet and water were available ad libitum. All animal procedures were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee and complied with guidelines outlined in the National Institute of Health Guide for the Care and Use of Laboratory Animals. The animals were anesthetized with halothane (2 %) in oxygen (flow rate: 1.0 l/min) during surgery prior to decerebration. The trachea was cannulated with a polyethylene tube (PE-240) to facilitate respiration. An indwelling i.t. catheter was placed according to the technique of Yaksh and Rudy [17]. A midline incision on the dorsal side of neck was made with a scalpel and the occipital crest of the skull was exposed with fine scissors. The atlanto-occipital membrane was then incised at the midline using the tip of an 18-gauge needle as a cutting edge. After detecting T13-vertebra at the most caudal rib, the distance between the occipital crest of the skull and the L1-vertebra where the L6-spinal segment is located was measured on each rat. A catheter (PE-10) was inserted through the slit and passed caudally to the L6-level of the spinal cord. At the end of the experiment, a laminectomy was performed to verify the catheter tip located at L6-segmental level.
Spinalization was performed in 7 rats by sectioning at the T8–9 level under halothane anesthesia. After the laminectomy, the dura and spinal cord were cut with scissors, and a sterile sponge (Gelform, The Upjohn Company, Kalamazoo, MI) was placed between the cut ends. The bladders of spinalized rats were manually expressed two or three times daily by applying pressure over the lower abdomen to release retained urine, and perigenital stimulation with cotton swab was performed to encourage reflex bladder emptying [9]. The experiments on spinalized rats were performed 3 to 4 weeks post-spinalization. In spinalized rats on the day of cystometric recording the T11–12 spinal vertebrae were removed and the dura mater was incised using the tip of a 30-gauge needle as a cutting edge to insert an i.t. catheter (PE-10) caudally to L6-level of the spinal cord.
Precollicular decerebration was performed according to a published method [11] that included ligating both carotid arteries followed by a removal of the forebrain using a blunt spatula. Halothane was then discontinued. Cotton and Avitene (MedChem Products Inc., Woburn, MA) were placed in the intracranial cavity and covered with agar. Experiments were started 2 h after the decerebration and conducted under unanesthetized conditions [21].
The urethral orifice was gently lifted with forceps and a bladder catheter (PE-50) was inserted transurethrally. The bladder catheter was connected to a pressure transducer to record the bladder pressure during cystometrograms (CMGs) when the bladder was filled with a constant infusion of physiological saline and allowed to empty around the catheter. Continuous infusion CMGs were performed using a constant, rapid infusion (0.21 ml/min) of saline into the bladder to elicit repetitive voidings, which allowed collection of data for a large number of voiding cycles [8]. The cystometric parameters measured were: bladder contraction amplitude (BCA), which is calculated by subtracting the lowest intraluminal pressure after the micturition from a peak micturition pressure; pressure threshold (PT), which is the intraluminal pressure to induce a voiding contraction; intercontraction interval (ICI), which is the time lag between two voiding cycles; bladder contraction duration (BCD), which is the interval between the beginning and end of a voiding contraction [8].
In all experiments, epoxy-coated stainless steel wire (50 μm, M.T Giken Co., Ltd., Tokyo, Japan) EMG electrodes were placed percutaneously in the external urethral sphincter (EUS) to examine synergy between bladder and EUS. This was performed using a 30-gauge needle with a hooked EMG electrode positioned at the tip. The needle was inserted into the sphincter approximately 5 mm lateral to the urethra and then withdrawn leaving the EMG wires embedded in the muscle [7]. The EMG activity was passed through a discriminator/ratemeter, and the output was recorded on a chart recorder. The peak activity (in pulses per second) during each micturition contraction was measured.
The effects of drugs on bladder contraction amplitude and duration, pressure threshold for inducing voiding, intercontraction interval [8], and EUS EMG activity were evaluated during a continuous CMG. Graded doses of a drug were given in each animal at 30–50 min intervals, depending on recovery time from the previous dose, to examine dose-response relationships. The effects of the drug on bladder and EUS EMG activity were evaluated by comparing values for 15 min between, before and after the administration. To obtain dose-response curves, the percentage changes between ‘before’ and ‘after’ drug injections were calculated and averaged for each dose. All values are expressed as mean ± S.E.M. Repeated measures analysis of variance (ANOVA) and paired t- test were used for statistical analysis and p<0.05 was considered significant.
Drugs used include: halothane (Ayerst Lab. Inc., Philadelphia, PA) and (+/−)-alpha-methyl-4-carboxyphenylglycine (MCPG, a group I/II mGluR antagonist). MCPG was dissolved in phosphate buffered saline, to make 1 μg/μl and 10 μg/μl concentration solutions. Single doses of the drug were administered in volumes of 3 to 10 μl followed by 7.5 μl flush with artificial CSF [6].
Results
In spinal cord intact rats, graded doses (3–100 μg) of MCPG were injected intrathecally (i.t.) at the L6 spinal segmental level. The 3 to 30 μg doses did not change EUS EMG activity (p=0.69 for 3 μg; p=0.19 for 10 μg; p=0.09 for 30 μg, paired t-test), whereas the 100 μg dose significantly increased the activity (p=0.02, paired t-test; p=0.02, repeated measures ANOVA) (Figs. 1 and 2, and Table 1). The facilitatory effect induced by 100 μg appeared within 3 min after the injection and persisted for 20–60 min (Fig. 1C). MCPG in 3–100 μg doses did not change any parameter of bladder activity (Figs. 1 and 2, and Table 1).
Fig. 1.
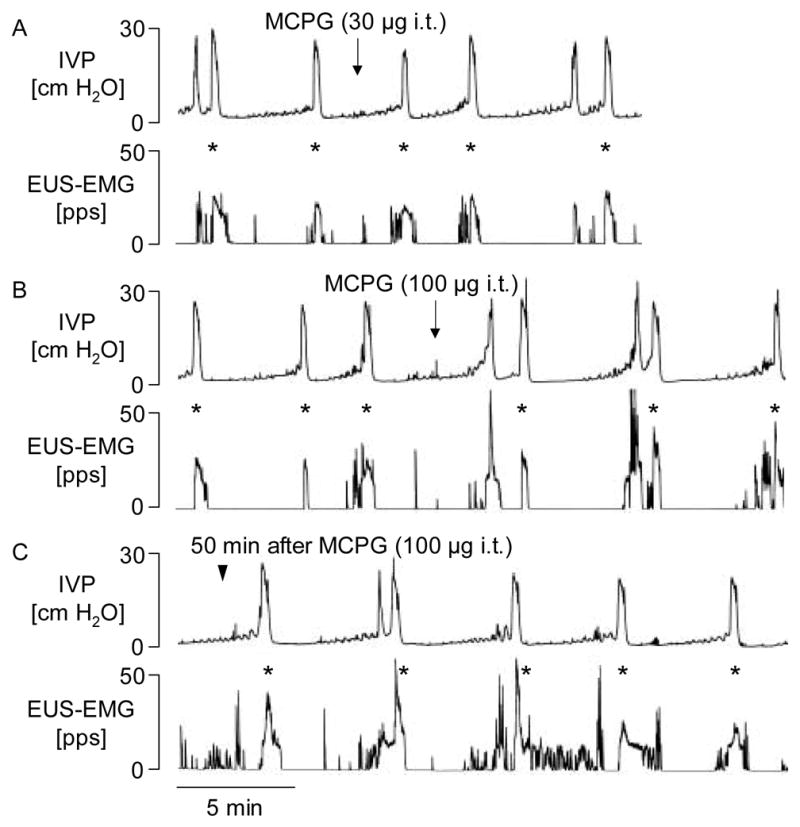
The effects of MCPG (30 and 100 μg i.t.) on the reflex bladder contractions and EMG activity of the external urethral sphincter (EUS) muscle during a continuous filling (0.21 ml/min) CMG in a spinal cord intact rat. Note that low dose (30 μg i.t.) of MCPG had no or a little effect on bladder and EUS EMG activity (panel A), whereas the drug at dose of 100 μg i.t. (panel B) markedly facilitated the EUS EMG activity during voiding but produced little change in the amplitude of bladder contractions. The facilitation in EUS EMG activity persisted for approximately 60 min in this rat (panel C). IVP, intravesical pressure; pps, pulses per second. Asterisks indicated the peak activity of EUS EMG evaluated for analysis.
Fig. 2.
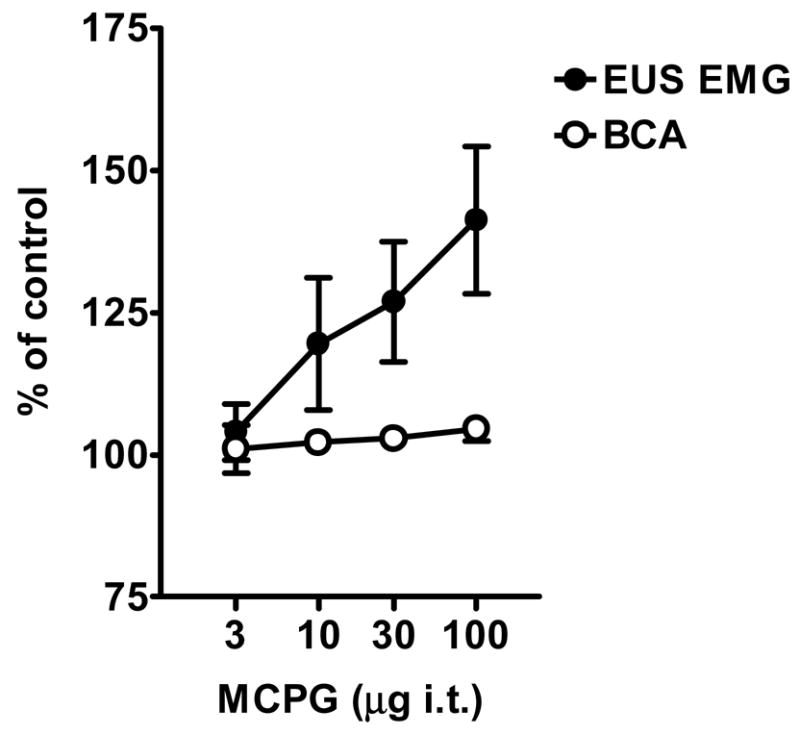
Dose-response curves showing the effects of MCPG (3, 10, 30 and 100 μg i.t.) on the bladder contraction amplitude (BCA) and peak external urethral sphincter electromyogram (EUS EMG) activity during continuous infusion CMGs in spinal intact rats. Data are presented as percent of control values (100 %) before drug administrations. All values are expressed as mean ± S.E.M. (n=6).
Table 1.
Effects of MCPG on cystometric parameters in spinal intact rats
| Dose of MCPG (i.t.) | BCA (cm H2O) | PT (cm H2O) | ICI (s) | BCD (s) | EUS EMG (pps) |
|---|---|---|---|---|---|
| 3 μg | |||||
| Before | 28.4 ± 2.7 | 7.4 ± 1.3 | 224 ± 46 | 37 ± 1 | 110 ± 33 |
| After | 28.5 ± 2.4 | 7.6 ± 1.5 | 224 ± 47 | 33 ± 3 | 113 ± 33 |
| 10 μg | |||||
| Before | 25.9 ± 1.0 | 8.0 ± 1.6 | 234 ± 54 | 31 ± 3 | 98 ± 31 |
| After | 26.4 ± 0.9 | 8.5 ± 1.4 | 240 ± 47 | 33 ± 3 | 107 ± 29 |
| 30 μg | |||||
| Before | 25.6 ± 1.0 | 7.2 ± 1.0 | 253 ± 52 | 33 ± 4 | 136 ± 37 |
| After | 26.4 ± 1.1 | 8.2 ± 1.6 | 273 ± 51 | 33 ± 3 | 165 ± 44 |
| 100 μg | |||||
| before | 26.4 ± 1.5 | 6.7 ± 0.8 | 283 ± 37 | 36 ± 3 | 126 ± 33 |
| After | 27.7 ± 2.0 | 7.2 ± 1.2 | 288 ± 35 | 35 ± 3 | 165 ± 35* |
All values are expressed as mean ± S.E.M. (n=6 for each parameter). Values of these parameters are compared between ‘before’ and ‘after’ injection of each dose (*p<0.05, repeated measures ANOVA and paired t-test). BCA, bladder contraction amplitude; PT, pressure threshold for inducing voiding; ICI, intercontraction interval; BCD, bladder contraction duration; EUS EMG, external urethral sphincter electromyogram expressed as peak activity in pulses per second (pps).
Baseline values of bladder and EUS EMG activity in spinal cord transected rats during continuous infusion CMGs before drug administrations were: 31.0 ± 3.3 cm H2O for BCA; 11.3 ± 2.1 cm H2O for PT; 31 ± 20 s for ICI; 46 ± 7 s for BCD; 151 ± 56 pps for EUS EMG). MCPG (3–100 μg i.t.) did not change any parameter of bladder or EUS EMG activity (data not shown).
Discussion
A previous immunocytochemical study of the rat lumbosacral spinal cord revealed mGluR5 immunoreactivity in parasympathetic preganglionic neurons and mGluR1 immunoreactivity in the external urethral sphincter (EUS) motor nucleus (i.e., Onuf’s nucleus) [1,2], raising the possibility that group I mGluRs (i.e., mGluR1 and mGluR5) are involved in the regulation of lower urinary tract function. Tanaka et al. reported that intrathecal administration of trans-(+/−)-1-amino-1,3-cyclopentanedicarboxylic acid (ACPD), a group I/II mGluR agonist, suppressed bladder contraction amplitude, intra-urethral pressure and EUS EMG activity in the rat, suggesting that spinal metabotropic glutamatergic mechanisms participate in an inhibitory control of bladder and EUS activity [12]. The present studies in rats with an intact spinal cord demonstrated that pharmacological blockade of mGluRs in the EUS motor nucleus and parasympathetic preganglionic neurons by MCPG facilitated EUS muscle activity during micturition with little effect on bladder contractions. On the other hand, in chronically spinal cord transected rats, MCPG did not alter activity of either the EUS EMG or the bladder. These results suggest that EUS EMG activity is tonically inhibited by reflex mechanisms involving spinal mGluRs and that the inhibition is dependent upon supraspinal pathways.
It has been well documented that peak EUS EMG activity in chronically-spinalized as well as spinal cord intact rats was markedly suppressed by i.t. administration of NMDA or AMPA receptor antagonists, suggesting that glutamatergic synaptic transmission via NMDA and AMPA receptors in the spinal cord plays an essential role in the excitatory control of EUS activity [21]. Thus, it is tempting to speculate that the spinal metabotropic glutamatergic mechanism is involved in the inhibitory modulation of the glutamatergic excitatory input to the EUS via the AMPA and/or NMDA receptors in spinal cord intact rats. On the other hand, the lack of effect of MCPG on EUS EMG activity in chronically-spinalized rats suggests that an mGluR-mediated inhibitory control of the EUS was eliminated after spinal cord transection, raising the possibility that removal of the inhibitory mechanism may contribute to uncoordinated activity of bladder and EUS (ie, detrusor-sphincter dyssynergia, DSD) that occurs after spinal cord injury.
More detailed analysis of EUS EMG recordings in rodents has revealed complex patterns during bladder filling and voiding. Tonic EUS EMG activity which occurs prior to micturition is switched to a bursting pattern (6–8 Hz) during voiding and then back to a tonic pattern at the end of voiding [7,10]. In rats the EUS EMG bursting activity creates a pumping action in the urethra that is essential for efficient voiding [10]. This pattern contrasts with the complete inhibition of EUS activity that occurs during micturition in humans. A recent study in mice lacking mGluR1 revealed that the EUS EMG activity during voiding exhibited a prominent tonic component superimposed on bursting activity during voiding [18]. These mice also exhibited a small decrease in voiding efficiency compared to voiding in wild type mice that had only bursting EUS EMG activity. A similar emergence of tonic EUS EMG activity and a reduction in the efficiency of voiding have been detected in anesthetized chronic spinal cord injured rats [3]. Although the pattern of EUS EMG activity was not analyzed in the present experiments it is possible that a similar emergence of tonic activity accounts for the increased peak EUS firing after treatment with MCPG. These studies suggest that a spinal mGluR1 mechanism in the sphincter motor nucleus plays an inhibitory role in the regulation of EUS activity during micturition and is necessary for efficient voiding.
In the present study, i.t. injections of MCPG did not alter the bladder activity in the storage phase (i.e., intercontraction interval) or the voiding phase (i.e., pressure threshold or bladder contraction amplitude and duration), suggesting that spinal mGluRs are not involved in sensory processing in the micturition reflex pathway and that mGluR in the parasympathetic preganglionic neurons (i.e., mGluR5) do not have a significant functional role at least in the rat model used in our experiments. On the contrary, a recent study using a whole-cell patch recording technique in a spinal slice preparation revealed that firing characteristics of lumbosacral parasympathetic preganglionic neurons were under mGluR modulatory control [5]. Furthermore, it has been reported that ACPD injected via an i.t. route suppressed the amplitude of bladder contractions [12]. However, our results imply that the spinal metabotropic glutamatergic mechanisms in bladder reflex pathways are ‘silent’ under physiological conditions.
The present data taken together with the results of previous studies, suggest that the spinal mGluRs (presumably, at least, mGluR1) are involved in inhibitory modulation of excitatory inputs (e.g., glutamatergic pathways via AMPA and/or NMDA receptors [21]) to the EUS. Thus, the mGluRs in the lumbosacral spinal cord may be a useful target for agonist and antagonist drugs to alleviate the lower urinary tract dysfunction.
Acknowledgments
This work was supported by National Institutes of Health Grant DK-49430 (W.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez FJ, Villalba RM, Carr PA, Grandes P, Somohano PM. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J Comp Neurol. 2000;422:464–487. doi: 10.1002/1096-9861(20000703)422:3<464::aid-cne11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Anneser JMH, Borasio GD, Berthele A, Zieglgänsberger W, Tölle TR. Differential expression of group I metabotropic glutamate receptors in rat spinal cord somatic and autonomic motoneurons: possible implications for the pathogenesis of amyotrophic lateral sclerosis. Neurobiol Dis. 1999;6:140–147. doi: 10.1006/nbdi.1999.0237. [DOI] [PubMed] [Google Scholar]
- 3.Cheng C-L, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 4.De Blasi A, Conn PJ, Pin J-P, Nicoletti F. Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol Sci. 2001;22:114–120. doi: 10.1016/s0165-6147(00)01635-7. [DOI] [PubMed] [Google Scholar]
- 5.Derjean D, Bertrand S, Nagy F, Shefchyk SJ. Plateau potentials and membrane oscillations in parasympathetic preganglionic neurons and intermediolateral neurons in the rat lumbosacral spinal cord. J Physiol (Lond) 2005;563.2:583–596. doi: 10.1113/jphysiol.2004.076802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldberg W, Fleischhauer K. Penetration of bromophenol blue from the perfused cerebral ventricles into the brain tissue. J Physiol (Lond) 1960;150:451–462. doi: 10.1113/jphysiol.1960.sp006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruse MN, Noto H, Roppolo JR, de Groat WC. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain Res. 1990;532:182–190. doi: 10.1016/0006-8993(90)91758-9. [DOI] [PubMed] [Google Scholar]
- 8.Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrograms in urethane-anesthetized rats. J Pharmacol Methods. 1986;15:157–167. doi: 10.1016/0160-5402(86)90064-1. [DOI] [PubMed] [Google Scholar]
- 9.Mallory B, Steers WD, de Groat WC. Electrophysiological study of micturition reflexes in rats. Am J Physiol. 1989;257:R410–R421. doi: 10.1152/ajpregu.1989.257.2.R410. [DOI] [PubMed] [Google Scholar]
- 10.Peng C-W, Chen J-JJ, Chang H-Y, de Groat WC, Cheng C-L. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn. 2006;25:388–396. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- 11.Sapru HN, Krieger AJ. Procedure for the decerebration of the rat. Brain Res Bull. 1978;3:675–679. doi: 10.1016/0361-9230(78)90016-3. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, Kakizaki H, Shibata T, Ameda K, Koyanagi T. Effects of a selective metabotropic glutamate receptor agonist on the micturition reflex pathway in urethane-anesthetized rats. Neurourol Urodyn. 2003;22:611–616. doi: 10.1002/nau.10138. [DOI] [PubMed] [Google Scholar]
- 13.Tsushihashi T, Abe I, Fujishima M. Role of metabotropic glutamate receptors in ventrolateral medulla of hypertensive rats. Hypertension. 1994;24:648–652. doi: 10.1161/01.hyp.24.6.648. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchihashi T, Averill DB. Metabotropic glutamate receptors in the ventrolateral medulla of rats. Hypertension. 1993;21:739–744. doi: 10.1161/01.hyp.21.5.739. [DOI] [PubMed] [Google Scholar]
- 15.Vitagliano S, Berrino L, Pizzirusso A, D’Amico M, Calderaro V, Maione S, Rossi F. Metabotropic glutamate receptors are involved in the control of breathing at the medulla oblongata level of anaesthetized rats. Neuropharmacology. 1994;33:859–864. doi: 10.1016/0028-3908(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 16.Wollmuth LP, Sobolevsky AI. Structure and gating of the glutamate receptor ion channel. Trends Neurosci. 2004;27:321–328. doi: 10.1016/j.tins.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 18.Yoshiyama M, Araki I, Beppu M, Du S, Kobayashi H, Zakoji H, Aiba A, Takeda M. Metabotropic glutamatergic receptor subtype 1 (mGluR-1) knockout mice exhibit detrusor-sphincter dyssynergia during micturition. International Continence Society 36th Annual Meeting; 2006; Abstract No. 210. [Google Scholar]
- 19.Yoshiyama M, de Groat WC. Supraspinal and spinalα-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and N-methyl-D-aspartate glutamatergic control of the micturition reflex in the urethane-anesthetized rat. Neuroscience. 2005;132:1017–1026. doi: 10.1016/j.neuroscience.2005.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshiyama M, Roppolo JR, de Groat WC. Effects of MK-801 on the micturition reflex in the rat – possible sites of action. J Pharmacol Exp Ther. 1993;265:844–850. [PubMed] [Google Scholar]
- 21.Yoshiyama M, Roppolo JR, de Groat WC. Effects of LY215490, a competitive α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J Pharmacol Exp Ther. 1997;280:894–904. [PubMed] [Google Scholar]
- 22.Yoshiyama M, Roppolo JR, Thor KB, de Groat WC. Effects of AMPA/kainate ( LY215490) and metabotropic (MCPG) glutamatergic receptor antagonists, on the micturition reflex in rats. Soc Neurosci Abstr. 1995;21:1873. [Google Scholar]


