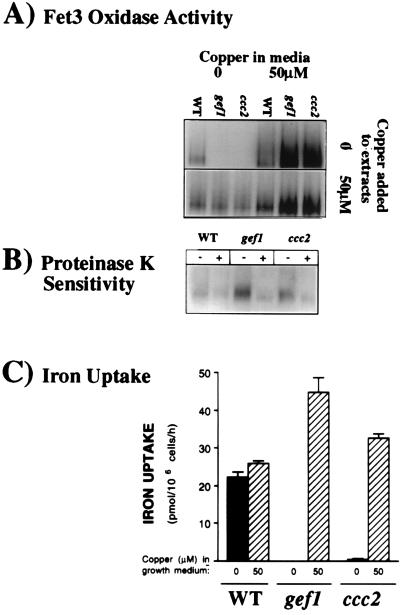
Figure 2.
Analysis of Fet3p function in gef1 cells. The copper status of Fet3p was characterized in congenic wild-type, gef1, and ccc2 cells by using assays of oxidase activity and high-affinity cellular iron uptake, previously shown to correlate with the presence of copper-loaded (holo-) Fet3p (15–17). (A) Oxidase assay. Cells were grown in YPD medium alone or with copper added. Duplicate samples were homogenized by using procedures that either preserve Fet3p in its apoprotein state or reconstitute Fet3p as a holoprotein, depending on the copper concentration in vitro (16). Solubilized membrane extracts (40 μg per lane) were separated in a nondenaturing gel and analyzed in situ for oxidase activity with o-dianisidine dihydrochloride as substrate. (B) Sensitivity of Fet3p to proteolytic digestion in intact cells. Cells grown in basal YPD medium as in A were divided into equal aliquots and either treated (+) with enzymes, zymolyase 100T and proteinase K or not (−). A proteinase inhibitor then was added to both treated and untreated samples, and these samples were chilled. The enzymes were also added to the untreated sample to make a control. This digestion protocol distinguishes cell surface from internal forms of Fet3p (15). Oxidase gels were then prepared as A, with copper added to membrane extracts to reconstitute holo-Fet3p. (C) High-affinity iron uptake assay. Uptake of radioactive iron was measured in cells grown as in A with procedures specific for high-affinity iron uptake (17).