Abstract
Finding a willing and suitable mate is critical for sexual reproduction. Visual, auditory, and chemical cues aid in locating and/or attracting partners. After mating, females from many insect species become less attractive. This is caused by changes in the quantity and/or quality of pheromones synthesized by the female and to changes in the female’s behavior. For example, female insects may stop releasing pheromones, assume a mate refusal posture, or move less in response to males. Many postmating changes in female insects are triggered by seminal fluid proteins from the male’s accessory gland proteins (Acps) and by sperm. To determine the role of seminal fluid components in mediating changes in attractiveness, we measured the attractiveness of Drosophila melanogaster females that had been mated to genetically altered males that lack sperm and/or Acps. We found that the drop in female attractiveness occurs in two phases. A short-term drop in attractiveness is triggered independent of the receipt of sperm and Acps. Maintenance of lowered attractiveness is dependent upon sperm.
Sexual reproduction involves conflicts that arise from competition for access to eggs or sperm. Typically females are the limiting resource. Males that gain the opportunity to copulate can maximize the probability that their sperm is used by preventing females from remating immediately. Males do this either by physically guarding females after mating or by inducing, via chemical or cellular cues, females to reject or to not attract other males (1). By using chemical methods, males are enabled to leave females after mating, giving those males the opportunity to increase their reproductive success by locating and inseminating additional females.
In many insect species, seminal fluid received from males during mating mediates postmating behavioral and physiological changes in females (reviewed in ref. 2 for Drosophila melanogaster). Seminal fluid is composed of sperm and the secreted products of the male’s accessory glands, ejaculatory duct, and ejaculatory bulb (3). Assays using mutant or transgenic D. melanogaster strains have shown that accessory gland proteins (Acps) trigger a rapid and transient increase in egglaying rate and decrease in sexual receptivity by the female (4), as well as aiding in sperm storage (4) and competition (5), and shortening the female’s life span (6). Persistence of elevated egglaying and depressed receptivity for up to 11 days postmating requires the presence of sperm in the female’s sperm storage organs (2).
Mating also causes female insects to become less attractive to male insects. In D. melanogaster, males are sexually stimulated by attractive female pheromones (7–9) and by the female’s movements (10, 11). The amount of courtship a female elicits from males is used as a measure of her attractiveness (12). Virgin females are vigorously courted by males whereas mated females elicit less courtship (13). After mating, the amount of attractive female pheromone (7,11-heptacosadiene) decreases and the activity level of the female diminishes (7, 9, 11). These changes cause the mated female to be a less stimulating visual and chemical cue to males. Mated females remain less attractive to males for 5–9 days after mating (7). Mating for only 3 of the normal 20 min is sufficient to cause females to be less attractive, but only for up to 4 h (7). During those first 3 min of mating, the male has transferred cuticular pheromones (predominantly 23-carbon tricosenes) onto his mate (8). On a female, these pheromones act as antiaphrodisiacs, lowering her level of attractiveness (8). The male-derived tricosenes, however, are rapidly lost from the female’s cuticle over the next few hours (8), suggesting that their effect is only transient. Thus, other stimuli from mating are necessary for the change in attractiveness to persist. These stimuli may act to regulate pheromone production by the mated female or to decrease the female’s activity level.
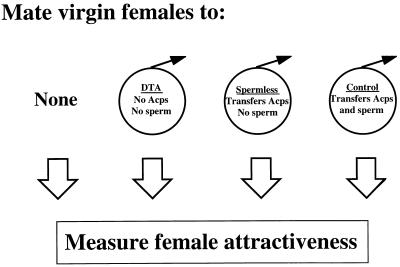
What triggers the drop in attractiveness is not known. Postmating regulation of pheromone production in female moths has been reported to be induced by either an accessory gland or testis-derived factor received from males during mating (14–17). We were interested in determining the relative contribution of Acps and sperm in regulating female attractiveness. To do this, we compared the attractiveness of females that had been mated to genetically altered D. melanogaster males that do not transfer sperm and/or Acps during mating (Fig. 1).
Figure 1.
Experimental design. Transgenic males lacking sperm and Acps, and mutant males lacking sperm were used to assess the role of these seminal fluid components in regulating female attractiveness. Virgin females were mated to DTA males (transfer neither Acps nor sperm), spermless males (transfer Acps only), or control males (transfer both). At 8, 10, or 24 h after the end of mating, the attractiveness of these mated females and that of age-matched virgin females were measured.
MATERIALS AND METHODS
Drosophila Strains.
Males lacking sperm only are the progeny of bw sp tud1 females mated to Canton S (CS) males (18). These spermless males produce and transfer normal quantities of Acps (19). Males lacking sperm and accessory gland main cell secretions (DTA) are transgenic animals. They were generated by directed cell ablation with the intracellular toxin diphtheria toxin subunit A driven by an accessory gland protein gene promoter (4). Because these males are sterile, the line is maintained by crossing transgenic females to nontransgenic ry506 (in CS background) males. As a result of this stock maintenance scheme, both DTA (ry+) and ry males result. ry males are used as the control in these experiments. Females used were from the CS stock.
Experimental Design.
Flies were raised on standard yeast-glucose food at 24°C on a 12/12-h light/dark cycle. Adults were collected within 4 h of eclosion, and males were separated from females under CO2 anaesthesia. Females were stored in vials of three to five individuals on yeasted medium. Males were stored individually. All flies were 4 days old when they were mated for the experiments. After mating, females were held individually in fresh food vials for 8, 10, or 24 h, at which time their attractiveness was measured. Each female was observed only once.
Female attractiveness was measured by aspirating, without anaesthesia, one female and one control male into a 0.2-cm3 Plexiglass observation chamber (7). Behavior was videotaped for 11–15 min. Total male courtship behavior (wing extension and vibration, attempted copulation) was recorded over 10 min, the observation period beginning once the two flies came into focus. In tests in which matings occurred, the courtship index was calculated as the percentage of time the male had spent courting before copulation began. All males used in the measurement of courtship indices received no prior exposure to mated or virgin females. The number of females examined was 36–57 per treatment group at each time point. Data were analyzed by one-way ANOVA followed by Fisher’s Protected Least Significant Difference on statview version 4.1.
RESULTS AND DISCUSSION
Initial Changes in Female Attractiveness.
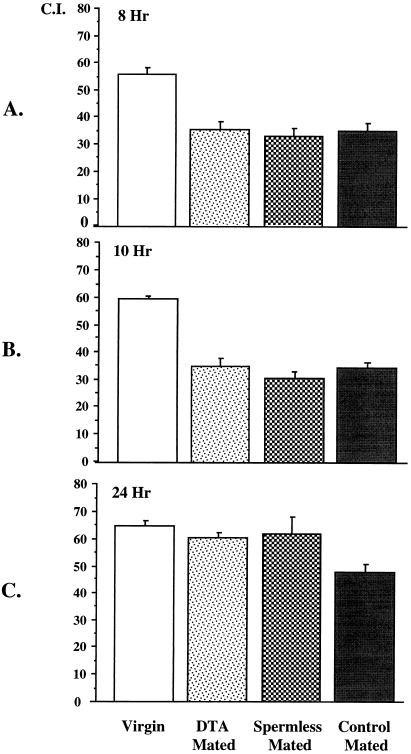
Because Acps exert their effects on behavior during the first 24 h postmating (3), we assessed the roles of Acps and sperm in regulating changes in female attractiveness only on the first day postmating. Morever, because male-derived antiaphrodisiacs are reported to influence female attractiveness for up to 6 h postmating (8), we confined our studies to after that time, so as to avoid interference from male-derived hydrocarbons. Accordingly, we measured and compared courtship indices at 8 and 10 h after mating, of females mated to DTA, spermless, or control males and of virgin females (Fig. 2 A and B). At both time points, all the mated females’ responses were similar. All mated females, whether or not they received Acps and/or sperm, were courted approximately half as vigorously as virgin females were. This demonstrates that Acps do not influence female attractiveness 8–10 h postmating, which is a time at which Acps have been demonstrated to mediate transitions in other postmating behaviors in the female (4). Our observations also indicate that the receipt of sperm does not influence female attractiveness at 8 and 10 h postmating, consistent with the report of Scott and Richmond (20).
Figure 2.
The effect of seminal fluid components on female attractiveness. Female attractiveness is indicated by a courtship index (C.I.), which is the percentage of a 10-min observation period that a naive control male spends courting the female (12). Mated females were tested at 8 (A), 10 (B), or 24 (C) h following a single mating. Virgin females for each time point tested were of the same age as the mated females. (A) At 8 h postmating, all mated females are significantly less attractive than virgin females (P < 0.0001). (B) At 10 h postmating, all mated females are still significantly less attractive than virgin females (P < 0.0001). (C) At 24 h postmating, only control mated females are significantly less attractive than virgin females (P < 0.0030). The attractiveness of females mated to DTA or to spermless males are not significantly different from that of virgin females (P = 0.41 and P = 0.65, respectively).
What aspect(s) of mating then trigger the initial drop in female attractiveness? Possible candidates include the receipt of seminal fluid products from the ejaculatory duct or the ejaculatory bulb. No fly strains exist that eliminate all products from these tissues, but the two products tested (esterase 6 from the ejaculatory duct, and cis-vaccenyl acetate from the ejaculatory bulb) do not individually cause the drop in female attractiveness (20, 21). Another suggested candidate is the male tricosenes that are transferred onto females during mating. Scott (8) has proposed that the passive transfer of male cuticular pheromones onto the cuticle of his mate causes the drop in attractiveness after the pair separates. However, the decrease in female attractiveness does not begin until approximately 20 min after the end of mating (22), which suggests that the transferred male pheromone alone is insufficient to cause a decrease in female attractiveness. Consistent with this, when we analyzed cuticular pheromones (as in ref. 23, in collaboration with L. Jackson) at 8 h postmating, we found that females mated to control males did not possess higher levels of 7-tricosene on their cuticles (P = 0.68) though they were significantly less attractive than virgin females. Females mated to control males also did not possess lower levels of 7,11-heptacosadiene than virgin females (P = 0.69).
Maintenance of Lowered Female Attractiveness.
The drop in female attractiveness is normally maintained for 5–9 days following mating (7). To determine whether Acps or sperm mediate the persistence of decreased female attractiveness, we compared the attractiveness of females mated to DTA, spermless, or control males at 24 h postmating. At 24 h after mating (Fig. 2C), only females mated to control males remained significantly less attractive than virgin females. The attractiveness of females mated to spermless or DTA males was not significantly different from one another or from virgin females. These results indicate that sperm is required for the persistence of lowered female attractiveness beyond the first 24 h postmating because only females receiving sperm (mates of control males) remained less attractive than virgin females. Our results also indicate that Acps do not regulate long-term female attractiveness because females receiving or not receiving Acps (mates of spermless or DTA males, respectively) are as attractive as virgin females.
By linking the presence of sperm in storage to her level of attractiveness, the female maximizes both her and her mate’s reproductive success (24, 25). For example, it protects the mated female from unwanted courters while she is fertile and laying eggs. Having to ward off courting males could be costly; it diverts the female from laying fertilized eggs and may even cause her to abandon a good oviposition site. Linkage of sperm to decreased female attractiveness is also advantageous for males. Female receptivity to male courtship is also dependent upon the female’s supply of stored sperm (26). Thus, males are prevented from courting mated females that are unreceptive toward their overtures. This is especially important because performing courtship behavior is sufficient to reduce male longevity (27).
How sperm regulates female attractiveness in D. melanogaster females is not known. We compared the pheromonal profile of virgin and mated females at 24 h postmating to determine whether sperm causes females to be less attractive by lowering the amount of 7,11-heptacosadiene the female exhibits or whether sperm stimulates females to biosynthesize more 7-tricosene. We found no differences in the amounts of either pheromone between virgin and mated females (P = 0.24 for 7-tricosene, and P = 0.2 for 7,11-heptacosadiene) at 24 h postmating. Possibly, the critical change that causes mated females to become less attractive is in her activity level in response to males. Mated females are less active in the presence of males (11). Males court moving females more vigorously than they do immobile females (10).
That changes in attractiveness are not correlative solely with pheromone levels in D. melanogaster differs from what has been demonstrated in moths (see ref. 28). In moths, the main determinant of female attractiveness is pheromone titer. Virgin females biosynthesize pheromones and release them to attract males. After mating, they cease synthesizing and releasing pheromones. Pheromone biosynthesis in female moths is stimulated by a brain factor called pheromone biosynthesis activating neuropeptide (PBAN) (reviewed in ref. 29). Male accessory gland and testis-derived factor(s) have been demonstrated to cause a depression in pheromone production by females. In the corn earworm Helicoverpa zea, a peptide (called pheromonostatic peptide) has been isolated from male accessory gland/duplex that, when injected into virgin females, causes the depletion of pheromones (17). In the tobacco budworm Heliothis virescens, injection of extracts of testes, but not of accessory glands, causes a significant decrease in pheromone production (14). How male seminal fluid components interact with PBAN to regulate pheromone production postmating is not known.
Conclusions.
Our results demonstrate that the change in female attractiveness in D. melanogaster occurs in two phases. Copulation, but not the receipt of Acps or sperm, is sufficient to initiate the transition from virgin to mated female level of attractiveness. The maintenance of decreased attractiveness requires sperm. The physiological basis of lowered attractiveness at 8 and 24 h postmating is not decreased quantities of attractive pheromone or increased levels of antiaphrodisiac. The critical change may be in the female’s behavior.
The importance of sperm in regulating female sexual attractiveness in insects appears to depend on the period of time that the female remains less attractive. Species whose females lose their attractiveness permanently or for extended periods of time utilize sperm to regulate their attractiveness, whereas species whose females become temporarily unattractive only do not. D. melanogaster females, which rely on sperm to regulate long-term attractiveness, are less attractive for up to 9 days after each mating, a substantial portion of their 5–6 week life span (6). Gypsy moth Lymantria dispar and brown-banded cockroach Supella longipalpa females, which also rely on sperm to regulate attractiveness, stop releasing pheromones permanently after mating (15, 30). In contrast, the female H. zea ceases to release pheromones for only one night after mating (31). This process is regulated by accessory gland products (16) that make up the spermatophore, and not by sperm.
The mechanism by which sperm exerts its effect is not known. Sperm may act by triggering receptors in the sperm storage organs; by interacting with female tissues to release a chemical that acts through the female’s circulatory system; or by secreting a substance itself (32, 33). In insects larger than Drosophila, it has been demonstrated, via surgical manipulation, that a sperm-mediated signal could act via the ventral nerve cord (15, 34). With Drosophila it will be possible to dissect in molecular terms, with the available genetics tools, how sperm exerts its effect on mated female behavior and physiology.
Acknowledgments
We thank members of the Wolfner lab, K. Fredrick, J. Liu, J. Lopez, M. Noor, and B. Studamire, for comments on the manuscript; L. Tompkins for helpful discussion and comments on the manuscript, experimental advice, and for lending the observation chambers; B. Johnson for use of his video setup; G. Churchill, M. Noor, and K. Wetterstrand for advice on statistics; and L. Jackson for allowing U.T. to come to his lab to analyze extracted pheromones. This work was supported by sequential National Science Foundation grants to M.F.W. (IBN 94-06171 and IBN 97-23356). U.T. was supported on a National Institutes of Health training grant (T32-GM07617) and a Sigma Xi Grant-in-Aid of Research.
ABBREVIATION
- Acps
accessory gland proteins
References
- 1.Thornhill R, Alcock J. The Evolution of Insect Mating Systems. Cambridge, MA: Harvard Univ. Press; 1983. [Google Scholar]
- 2.Wolfner M F. Insect Biochem Mol Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- 3.Bairati A. Monit Zool Ital. 1968;2:105–182. [Google Scholar]
- 4.Kalb J M, DiBenedetto A J, Wolfner M F. Proc Natl Acad Sci USA. 1993;90:8093–8097. doi: 10.1073/pnas.90.17.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harshman L, Prout T. Evolution. 1994;48:758–766. doi: 10.1111/j.1558-5646.1994.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 6.Chapman T, Liddle L F, Kalb J M, Wolfner M F, Partridge L. Nature (London) 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 7.Tompkins L, Hall J C. J Insect Physiol. 1981;27:17–21. [Google Scholar]
- 8.Scott D. Proc Natl Acad Sci USA. 1986;83:8429–8433. doi: 10.1073/pnas.83.21.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferveur J-F. BioEssays. 1997;19:353–358. doi: 10.1002/bies.950190413. [DOI] [PubMed] [Google Scholar]
- 10.Tompkins L, Gross A C, Hall J C, Gailey D A, Siegel R W. Behav Genet. 1982;12:295–307. doi: 10.1007/BF01067849. [DOI] [PubMed] [Google Scholar]
- 11.Scott D, Richmond R C, Carlson D A. Anim Behav. 1988;36:1164–1173. [Google Scholar]
- 12.Tompkins L, Hall J C, Hall L M. J Insect Physiol. 1980;26:689–697. [Google Scholar]
- 13.Bastock M, Manning A. Behavior. 1955;8:85–111. [Google Scholar]
- 14.Ramaswamy S B, Mbata G N, Cohen N E, Moore A, Cox N M. Arch Insect Biochem Physiol. 1994;25:301–315. doi: 10.1002/arch.940250406. [DOI] [PubMed] [Google Scholar]
- 15.Giebultowicz J M, Raina A K, Uebel E C, Ridgway R L. Arch Insect Biochem Physiol. 1991;16:95–105. [Google Scholar]
- 16.Raina A K. J Insect Physiol. 1989;35:821–826. [Google Scholar]
- 17.Kingan T G, Thomas-Laemont P A, Raina A K. J Exp Biol. 1993;183:61–76. [Google Scholar]
- 18.Boswell R E, Mahowald A P. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 19.Bertram M J, Akerkar G A, Ard R L, Gonzalez C, Wolfner M F. Mech Dev. 1992;38:33–40. doi: 10.1016/0925-4773(92)90036-j. [DOI] [PubMed] [Google Scholar]
- 20.Scott D, Richmond R C. Anim Behav. 1985;33:817–824. [Google Scholar]
- 21.Vander Meer R K, Obin M S, Zawistowski S, Sheenhan K B, Richmond R C. J Insect Physiol. 1986;32:681–686. [Google Scholar]
- 22.Bubis, J. A., Degreen, H. P., Unsell, J. L. & Tompkins, L. (1998) Anim. Behav., in press. [DOI] [PubMed]
- 23.Scott D, Jackson L L. J Insect Physiol. 1988;34:863–871. [Google Scholar]
- 24.Manning A. Anim Behav. 1967;15:239–250. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- 25.Forsberg J, Wiklund C. Behav Ecol Sociobiol. 1989;25:349–356. [Google Scholar]
- 26.Gromko M H, Markow T. Anim Behav. 1993;45:253–262. [Google Scholar]
- 27.Cordts R, Partridge L. Anim Behav. 1996;52:269–278. [Google Scholar]
- 28.Roelofs W L. Proc Natl Acad Sci USA. 1995;92:44–49. doi: 10.1073/pnas.92.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raina A K, Menn J J. Arch Insect Biochem Physiol. 1993;22:141–151. doi: 10.1002/arch.940220112. [DOI] [PubMed] [Google Scholar]
- 30.Smith A F, Schal C. J Insect Physiol. 1990;36:369–373. [Google Scholar]
- 31.Raina A K, Klun J A, Stadelbacker E A. Ann Entomol Soc Am. 1986;79:128–131. [Google Scholar]
- 32.Manning A. Nature (London) 1962;194:252–253. [Google Scholar]
- 33.Mbata G N, Ramaswamy S B. Physiol Entomol. 1990;15:423–432. [Google Scholar]
- 34.Foster S P. J Insect Physiol. 1993;39:267–273. [Google Scholar]