Figure 1.
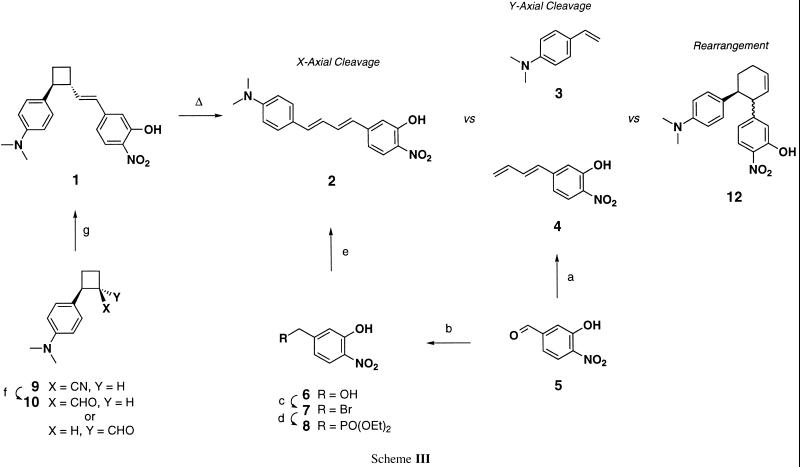
Reaction a: allyltriphenylphosphonium bromide, n-butyllithium, THF, 0°C to room temperature (RT), 3 h, 94% yield of a 1:1 mixture. Reaction b: NaBH4, MeOH/THF/Et2O (5:5:1), 0°C to RT, 97% yield. Reaction c: CBr4, PPh3, 4-Å molecular sieves, CH2Cl2, 0°C to RT, 86% yield. Reaction d: P(OEt)3, dimethylformamide (DMF), 155°C, 1.5 h, 99% yield. Reaction e: (i) sodium hexamethyldisilazide (NaHMDS) (2 eq), DMF, THF, 0°C to RT, 1 h; (ii) N,N-dimethylaminocinnamaldehyde, THF, −20°C to RT, 6 h, 55% yield. Reaction f: (i) diisobutylaluminum hydride (DIBAL-H) (3.5 eq), PhCH3, −78°C, 45 min; (ii) quench with 10% HOAc/H2O, −78°C to RT, 1 h, 68% yield of a 1:1 mixture. Reaction g: (i) 8, NaHMDS, DMF, THF, 0°C to RT, 1 h; (ii) add 10 in THF, 6 h, 0°C to RT, 4 h, 89% yield.