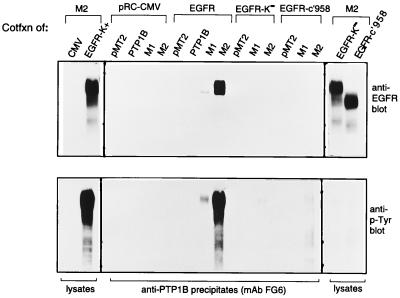
Figure 4.
Reconstitution of the interaction between D181A and the EGFR requires EGFR autophosphorylation. 293 cells were cotransfected with plasmids expressing PTP1B, the C215A or D181A PTP1B mutants, and plasmids expressing the human EGFR or a catalytically inactive (K−) mutant, in which the Lys responsible for coordinating ATP, Lys-721, was converted to Arg, or a C-terminally truncated (c′958) form that lacks all the major autophosphorylation sites. Immunoprecipitates of PTP1B were split in half and resolved by SDS/PAGE on duplicate gels. One gel (Upper) was blotted with anti-EGFR antibody KSM and the other (Lower) was blotted for pTyr with G98. Panels on each end represent immunoblots of 50 μg of lysate from the cells cotransfected with D181A-PTP18 (M2) and the various EGFR forms. CMV, cytomegalovirus. Although both the K− and c′958 forms of EGFR are expressed well, neither contains pTyr, and both fail to interact with the D181A mutant of PTP1B.