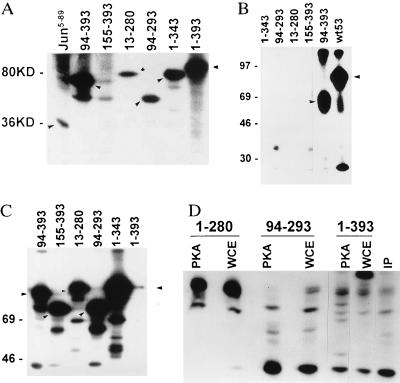
Figure 1.
(A) Effect of WCE kinases on phosphorylation of p53. GST-p53 wild-type (amino acids 1–393; molecular mass 80 kDa) or truncated forms (as indicated by arrows) were incubated with WCE as source of kinases. The solid-phase kinase reaction initiated by addition of [γ-32P]ATP for 15 min at room temperature, followed by washes of glutathione beads-bound GST-p53 and elution of p53 from beads by SDS-loading buffer. Samples were separated on SDS/15% PAGE and autoradiographed. Molecular masses are indicated at left in kDa. GST-c-jun5–89 used as control is shown in the left lane. (B) CKII phosphorylation of p53. Solid-phase kinase assay with respective GST-p53 proteins (position of major phosphorylation site indicated by arrow) was performed in the presence of 40 ng of the catalytic subunit of CKIIβ. (C) PKA phosphorylation of p53. GST-p53 proteins used for solid-phase kinase reaction using catalytic subunit of PKA (80 ng) as source of kinase. Position of major phosphorylation site is indicated by arrows. (D) Mapping of PKA phosphorylation sites on p53. Products of solid-phase kinase reaction using PKA (2 μg of enzyme per reaction) or WCE (2 μg) were washed and then separated on SDS/PAGE. Pattern of endogenous p53 phosphorylation is shown in IP lane, in which cells were labeled with [32P]-orthophosphate (100 μCi) for 4 h before p53 was immunoprecipitated (200 ng of p53 antibody per 1 mg of protein). Bands corresponding to each of the GST-p53 proteins were excised and subjected to trypsinization followed by separation on an isoelectric focusing gel (pH range 3.5–10). Peptides were analyzed by autoradiography.