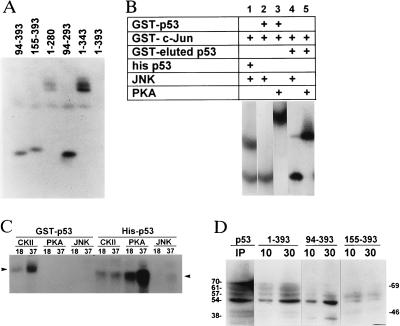
Figure 2.
(A) JNK phosphorylation of GST-p53 proteins. Full-length or truncated forms of GST-p53 proteins were used for solid-phase kinase reaction using JNK purified from WCE. Respective bands were excised, subjected to digestion with trypsin, and analyzed further by separation on isoelectric focusing gel. (B) p53 phosphorylation by JNK. Solid-phase kinase reactions were performed using bacterially produced active JNK (200 ng) or PKA (2 μg) as the source of kinase and GST p531–393 (lanes 2, 3), thrombin-digested p53 that lacks the GST portion (lanes 4, 5), Hisp53 (lane 1), or GST c-jun5–89 (lanes 1–3) as substrates. (C) CKIIβ, PKA, and JNK phosphorylation of Hisp53 and GST-p53. GST-p53 or Hisp53 substrates were used in solid-phase kinase reaction using CKIIβ (40 ng), PKA (80 ng), or JNK (200 ng) at 18° or 37°C. Arrows point to the position of the GST or histidine-tagged protein. (D) JNK binding to p53. GST-p53 constructs were incubated with protein extracts for either 10 or 30 min at room temperature before beads-bound material was washed and analyzed on Western blots using antibodies to JNK. IP lane represents proteins immunoprecipitated with antibodies to p53 and analyzed by Western blotting with antibodies to JNK. Molecular mass (kDa)is shown at the right, and molecular masses of proteins recognized by JNK antibodies are indicated on the left.