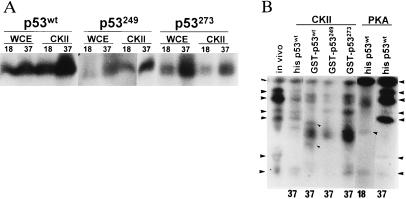
Figure 3.
(A) Effect of temperature on phosphorylation of wild-type and mutant forms of p53. GST-p53 was subjected to solid-phase kinase reaction using the kinases at 18°C or 37°C. (B) Mapping of mutant p53 phosphorylation. Products of solid-phase p53 phosphorylation at indicated temperatures were separated on SDS/PAGE, and p53 bands, identified through autoradiography, were cut, excised, and digested with trypsin before separation on an isoelectricfocusing gel. Resulting phosphorylated peptides are indicated by the arrowheads on the sides. Phosphorylated peptides identified in wild-type, but not mutant, forms of p53 are indicated by arrowheads on lane 3 (compare with lanes 4 and 5).