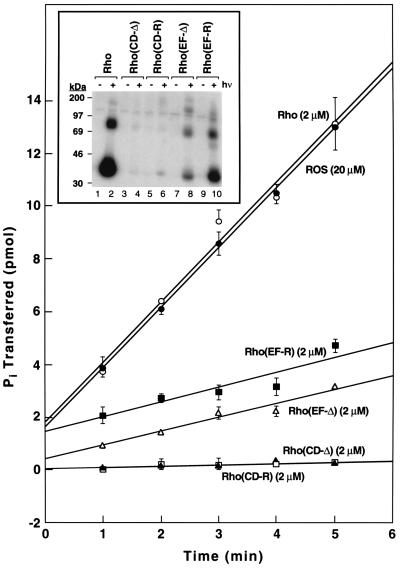
Figure 3.
Comparative rates of phosphorylation of rhodopsin mutants by RK. In the control experiments, rhodopsin concentration was 2 μM while ROS membranes were used at 20 μM rhodopsin concentration. The incorporation of phosphate from [γ-32P]ATP was measured by nitrocellulose filter binding and quantitated by Cerenkov counting. The assays measured initial rates of phosphorylation. All assays (Materials and Methods) used 61 ng RK of standard specific activity. (Inset) Analysis by gel electrophoresis of phosphorylation of rhodopsin mutants at 10 μM substrate concentration except for WT rhodopsin, which was at 2 μM concentration. Shown are phosphorylation reactions of WT rhodopsin, CD-Δ, CD-R, EF-Δ, and EF-R in the dark and after illumination. Each assay used 61 ng of RK of standard specific activity. To facilitate separation by SDS/PAGE the proteins were treated with PNGase F for deglycosylation before loading onto the gel (Materials and Methods). The incorporation of phosphate from [γ-32P]ATP was measured by phosphoimaging.