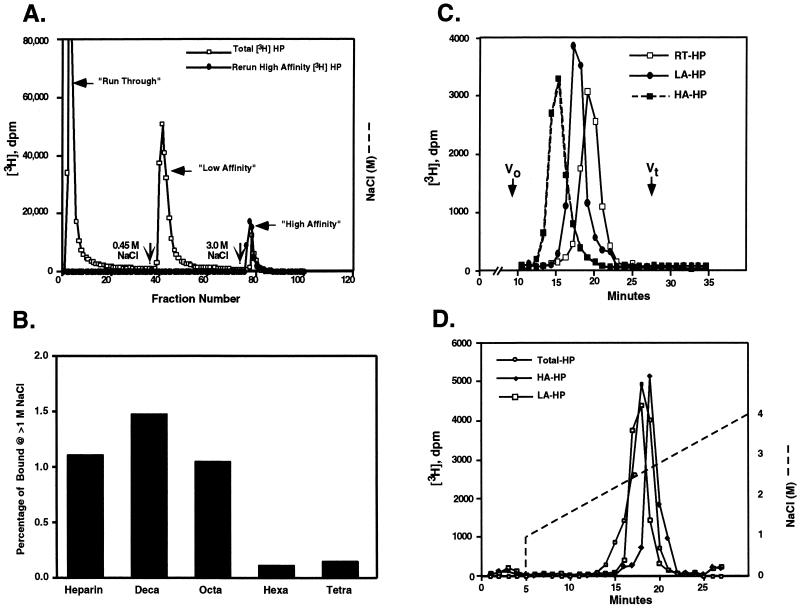
Figure 1.
HIP peptide affinity chromatography of Hep and Hep oligosaccharides. (A) [3H]Hep dissolved in 0.15 M NaCl/PBS, pH 7.4, was applied to a 1-ml HIP peptide affinity FPLC column equilibrated with the same buffer (□). The column then was eluted first with 0.15 M NaCl then with 0.45 M or 3.0 M NaCl in PBS (indicated by vertical arrows) at a flow rate of 0.5 ml/min to generate RT-, LA-, and HA-Hep fractions, respectively. Rechromatography of the high-affinity fraction (•) demonstrated quantitative rebinding and elution in the 3.0 M NaCl eluate. Each fraction (0.5 ml per fraction) was collected and radioactivity determined by liquid scintillation counting. (B) [3H]Hep oligosaccharide or [3H]Hep was resuspended in 0.15 M NaCl/10 mM phosphate, pH 7.4, and loaded onto a 1-ml HIP peptide affinity FPLC column equilibrated with 0.15 M NaCl/10 mM phosphate, pH 7.4. The column then was washed with 1.0 M NaCl/10 mM phosphate, pH 7.4, extensively. Finally, the column was eluted with 3.0 M NaCl/10 mM phosphate, pH 7.4. The percentage of [3H]Hep or [3H]Hep oligosaccharide binding to the column and eluting at >1.0 M NaCl is shown. (C) Run-through [3H]Hep (RT-Hep, □), low-affinity [3H]Hep (LA-Hep, •), and high-affinity [3H]Hep (HA-Hep, ▪) isolated as described above were analyzed on a Superose 12 column. The elution positions of blue dextran (Vo) and [3H]glucosamine (Vt) standards are indicated. (D) Bulk [3H]Hep (○), LA-Hep (□), and HA-Hep (♦) were analyzed by Mono Q anion exchange liquid chromatography column. The NaCl gradient conditions are indicated by a dashed line.