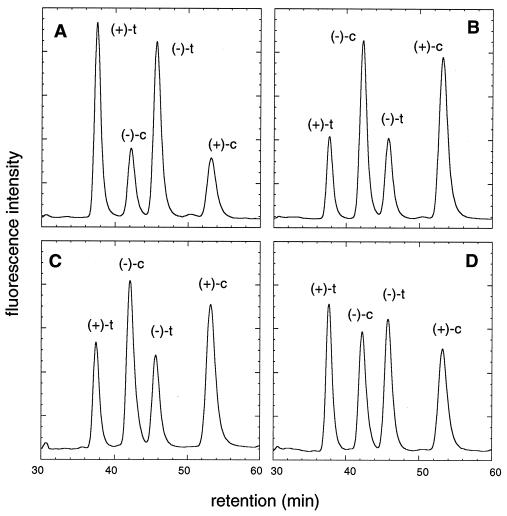
Figure 2.
Reverse-phase HPLC chromatograms of dAdo adducts formed by alkylation with (±)-anti-BPDE or its racemic chlorohydrin derivative (trans-BPDCH). (A) Adducts formed with the epoxide in the absence of chloride. (B) Adducts formed with the epoxide in the presence of 175 mM NaCl. (C) Adducts formed with trans-BPDCH in the absence of chloride. (D) Co-injection of the samples used in A and C. Peaks in A are labeled with the enantiomer of BPDE from which the adduct was derived [(+), RSSR; (−), SRRS] and with the epoxide ring opening geometry (c = cis; t = trans). Adducts were formed at room temperature in 10 mM sodium cacodylate buffer (pH 7.0) containing 5% THF (vol/vol), and analyzed by reverse-phase HPLC using a 1:1 methanol/water mobile phase and fluorescence detection.