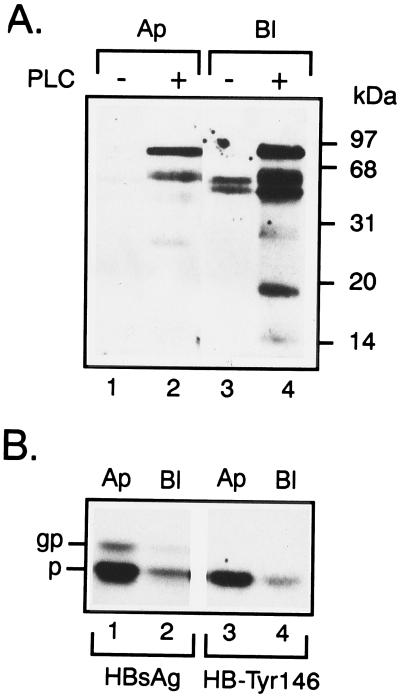
Figure 3.
Permanently transfected FRT cells still distribute most of their GPI-anchored proteins to the basolateral domain but secrete HBsAg apically. (A) FRT cells transfected with the pSV2-TKneo-HBsAg plasmid and selected by neomycin resistance were analyzed by domain-selective biotinylation of apical (lanes 1 and 2) and basolateral (lanes 3 and 4) surface proteins. Triton X-114 extraction and temperature-induced phase separation, followed by PL-PLC (6 units/ml) treatment of the detergent phase, distinguishes the GPI-proteins by their PI-PLC-induced partitioning shift into the aqueous phase (lanes 2 and 4). Biotinylated proteins were resolved by SDS/PAGE, electroblotted onto nitrocellulose filters, and revealed by streptavidin-horseradish peroxidase and enhanced chemiluminiscence detection. After PI-PLC treatment, the aqueous phase showed most GPI-proteins predominantly basolateral (compare lanes 2 and 4); (B) Fluorograms show the preferential apical secretion of both the HBsAg (lanes 1 and 2) and HB-Tyr146 mutant (lanes 3 and 4) after pulse–chase labeling and immunoprecipitation.