Figure 1.
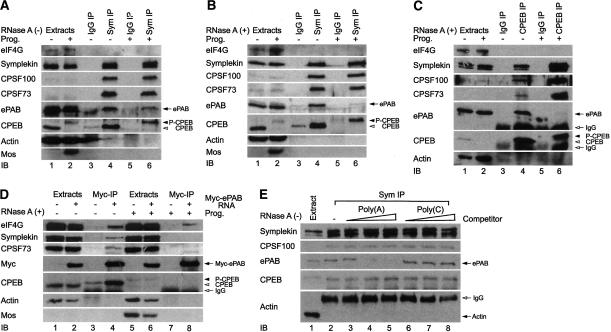
ePAB interacts with the cytoplasmic polyadenylation machinery. Extracts from oocytes, some of which were incubated with progesterone to induce maturation, were immunoprecipitated (IP) with symplekin antibody or control mouse IgG in the presence of RNase inhibitor (100 U/mL) (A) or RNase A (100 μg/mL) (B). Immunoblots of the precipitates were probed for eIF4G, symplekin, CPSF100, CPSF73, ePAB, CPEB, and, as negative controls, actin and mos. (P-CPEB) Phospho-CPEB. (C) Extracts from untreated or progesterone-treated oocytes were subjected to IP in the presence of RNase A with CPEB antibody or control rabbit IgG, which was followed by immunoblotting. (D) mRNA encoding myc-ePAB was injected into oocytes; following 12 h incubation, some of them were incubated with progesterone to induce maturation, and the oocytes were lysed and subjected to IP with myc-specific antibody (9E10). The coprecipitating proteins were analyzed on immunoblots. (E) Extracts from eggs (no RNase A) were subjected to a symplekin IP in the absence or presence of increasing amounts (0.1, 0.5, and 2.5 μg) of poly(A) or poly(C). The coprecipitating proteins were analyzed on immunoblots.