Fig. 1.
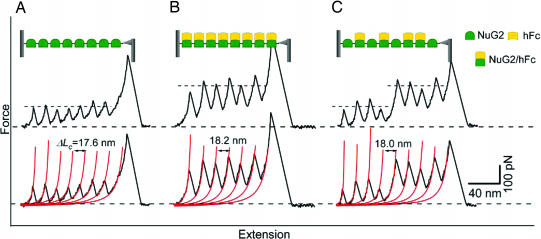
Mechanical stability of NuG2 is a functional reporter for the binding of hFc. (A) Stretching polyprotein (NuG2)8 results in typical sawtooth-like force–extension curves that are characterized by unfolding forces of ≈105 pN and contour length increments ΔLc of ≈18 nm. Each individual force peak corresponds to the mechanical unfolding of individual NuG2 domains in the polyprotein. All of the NuG2 domains unfold at a similar force of ≈105 pN, as indicated by the dashed line. Red lines correspond to the WLC fits to the force–extension curve with ΔLc of 17.6 nm. (B) The mechanical stability of NuG2 is enhanced by the binding of hFc. When preequilibrated with 33.3 μM hFc, the majority of NuG2 domains unfold at much higher forces of ≈210 pN, indicating that the unfolding force of NuG2 can be used as an indicator to report effective hFc binding to NuG2. Red lines correspond to the WLC fits to the force–extension curve with ΔLc of 18.2 nm. (C) Force–extension curves of NuG2 at an intermediate concentration of hFc directly identify the hFc-bound and hFc-free forms of NuG2 at the single-molecule level. When preequilibrated with 17.8 μM hFc, the unfolding forces of NuG2 occur at two distinct levels (as indicated by the dashed lines): the first four unfolding events occurred at ≈105 pN and can be ascribed to the unfolding of hFc-free NuG2; the last four unfolding events occurred at ≈210 pN, which corresponds to the unfolding of hFc-bound NuG2. Red lines correspond to the WLC fits to the experimental data. (Insets) Schematic illustration of the stretching of (NuG2)8 polyprotein between an AFM tip and glass substrate in the absence or presence of hFc. The functional states of NuG2 domains in the polyprotein also are indicated.