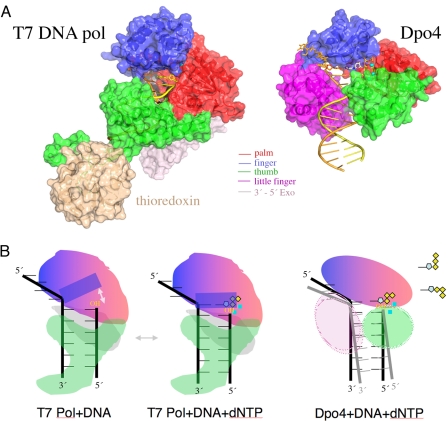
Fig. 2.
Structural comparison of T7 DNA polymerase (A-family) (PDB ID code 1T7P) and Dpo4 (Y-family) (PDB ID code 2AGQ). (A) The polymerase domain is shown in the same colors as in Fig. 1. Thioredoxin (wheat) enhances the processivity of T7 DNA pol. DNA is shown in yellow (primer) and brown (template) tubes, the two metal ions as cyan spheres, and the incoming nucleotide (only visible in Dpo4) as silver and multicolored sticks. (B) Diagrams of the conformational change of a helix (solid blue rectangle) in the finger domain of T7 DNA pol upon binding of a correct incoming nucleotide (dNTP). Movement of the helix is indicated by a gray arrow. The reactants and catalysts are snug in the closed active site. (C) Illustration of the flexible LF and thumb domains of Y-family polymerases, which facilitate the movement of the template–primer duplex. The spacious and open active site also allows multiple conformations of the dNTP (as diagramed in the upper right corner) and makes it difficult to align the 3′-OH of the primer strand, dNTP, metal ions, and catalytic carboxylates.