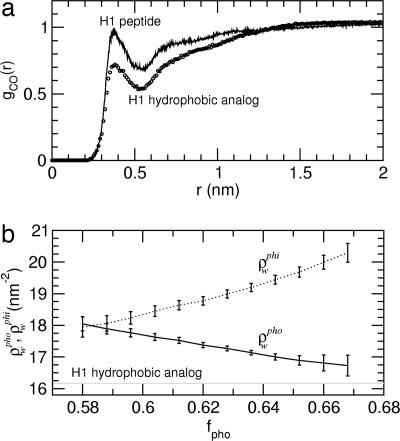
Fig. 3.
Hydration properties. (a) Radial distribution, gCO(r), of the distance, r, between the water oxygen atoms and the peptide surface hydrophobic-residue carbon atoms (circles, H1 hydrophobic analog; solid line, H1 peptide calculated for structures with ρwpho ≤ 16.75 nm−2). (b) H1 peptide hydration density around hydrophobic, ρwpho (solid line), and hydrophilic, ρwphi (dotted line), residues as a function of the fraction of the solvent-accessible surface area that is hydrophobic, fpho. Nearly identical results are found when Spho is used instead of fpho. ρwpho (ρwphi) is defined here as the number of water molecules, Nw, within a 0.55-nm (0.45-nm) radius cutoff from every hydrophobic (hydrophilic) atom on the peptide surface per unit of exposed hydrophobic (hydrophilic) surface area (ρwpho = Nw/Spho; ρwphi = Nw/Sphi). ρwpho was also calculated for the H1 hydrophobic analog, and the average value is shown as a gray line.