Figure 2.
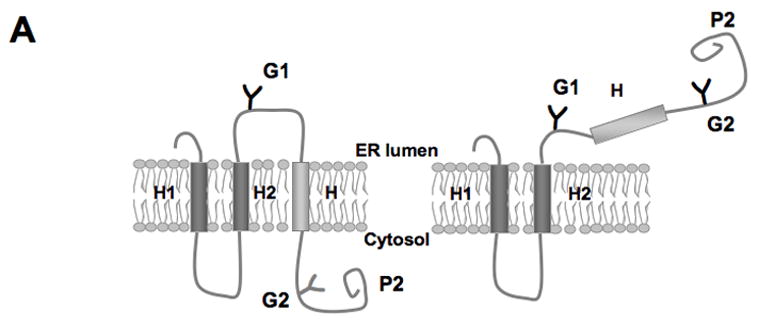
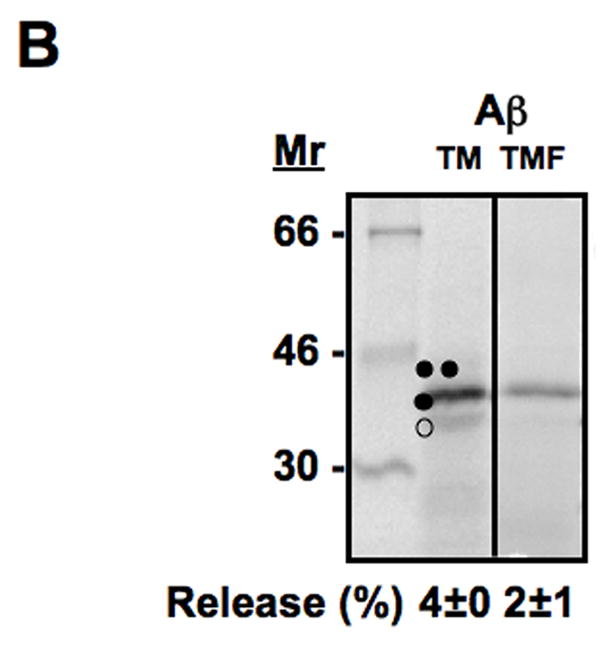
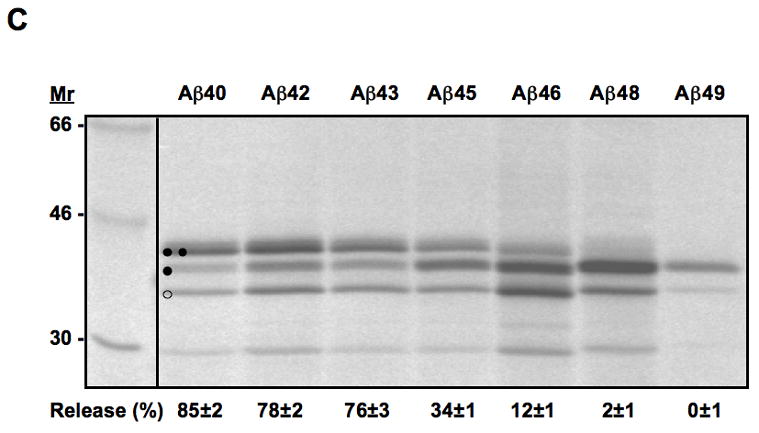
Translocon-mediated membrane insertion of different Aβ and CTF constructs. (A) The leader peptidase model protein. Wild-type leader peptidase (Lep) has two transmembrane helices (H1, H2) and a large lumenal domain (P2). It inserts into rough microsomes in an Nlum–Clum orientation. In the constructs reported here, Aβ 40–49 and CTF C50–59 segments (see Table S1) were inserted into the P2 domain in the position indicated (H, gray rectangle), and Asn-X-Thr glycosylation acceptor sites (G1, G2) were introduced on both sides of the Aβ and CTF segments. Constructs in which the Aβ or CTF segment is integrated into the endoplasmic reticulum (ER) membrane become glycosylated only on the G1 site (left), whereas those in which the segment is translocated across the ER membrane become glycosylated on both the G1 and G2 sites (right). (B) In vitro translation in the presence of dog pancreas rough microsomes (RMs) of constructs containing the Aβ transmembrane segment with (lane TMF) and without (lane TM) additional flanking residues from APP (see Table S1). (C) In vitro translation in the presence of dog pancreas rough microsomes of constructs containing the indicated Aβ segments. Unglycosylated, singly glycosylated, and doubly glycosylated forms of the protein are indicated by one open circle, one filled circle and two filled circles, respectively. The percentage of molecules not retained in the membrane is given below the lanes (average and standard deviation of at least three independent experiments).
