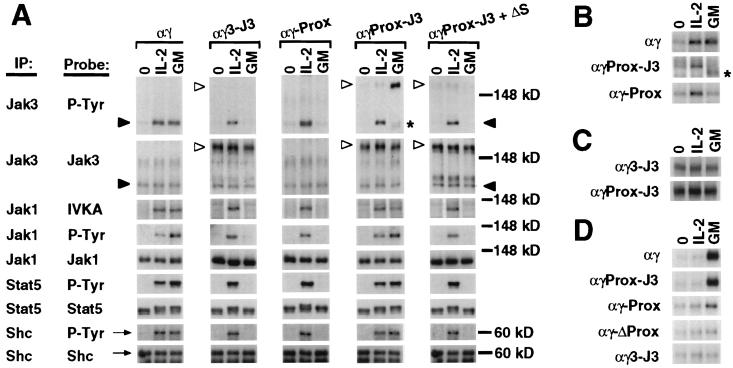
Figure 3.
Tyrosine phosphorylation and Jak kinase activation mediated by chimeric receptor chains or the endogenous IL-2R in the T cell line CTLL-2. Cells coexpressing ββ and the indicated derivative of αγ or coexpressing ββ–ΔS and αγPROX–J3 were deprived of IL-2 for 4 h and then stimulated with media alone (0), human IL-2 (100 units/ml), or human GM-CSF (GM; 100 ng/ml) for 10 minutes at 37°C. Cells were lysed, and cytoplasmic fractions were subjected to immunoprecipitation (IP) with antibodies to the indicated proteins. Immunoprecipitated proteins were either probed by Western blot analysis with the indicated antibodies (A and D) or subjected to an in vitro kinase assay (IVKA) and visualized by autoradiography (A-C). The asterisks in A and B indicate a band in the GM lane of αγPROX–J3 corresponding to phosphorylated ββ, which coprecipitates with αγPROX–J3 in the presence of GM-CSF. (A) Tyrosine phosphorylation of Jak3, Jak1, Stat5, and Shc [assessed by probing with anti-phosphotyrosine antibody (P-Tyr)] and catalytic activation of Jak1 (assessed by IVKA). Endogenous Jak3 (closed arrowheads) can be distinguished from the chimeric forms of Jak3 (open arrowheads) by molecular mass. (B) Autoradiogram showing in vitro kinase activity of endogenous Jak3 immunoprecipitated with anti-Jak3 antiserum from cells coexpressing ββ and the indicated derivative of αγ. (C) Autoradiogram showing in vitro kinase activity of the Jak3 component of the chimeric chains αγ3–J3 and αγPROX–J3 immunoprecipitated with an mAb to the extracellular domain of human GM-CSFRα. (D) Tyrosine phosphorylation of the chimeric ββ chain in cells coexpressing the indicated derivative of αγ. Phosphorylation of ββ was assessed by immunoprecipitating with an antibody to phosphotyrosine and probing with antiserum to the cytoplasmic domain of human IL-2Rβ, rather than the converse procedure, because this allowed better visualization of low level phosphorylation events. This antiserum does not recognize endogenous murine IL-2Rβ by immunoblot. The phosphotyrosine content of ββ was confirmed upon reprobing with antibody to phosphotyrosine; the presence of constitutively phosphorylated proteins on these second blots allowed confirmation of equivalent loading of samples (data not shown).