Abstract
A new biomaterial is presented which consists of a cellulose derivative - silanised hydroxyethylcellulose (HEC-SIL) and biphasic calcium phosphate (BCP). Rheological properties of the polymer itself and its mixture with BCP are pH dependent. At pH = 10–12 HEC-SIL is liquid and undergoes quick gellation at pH < 9. Similarly the paste of HEC-SIL and BCP is fluid and injectable at higher pH and solidifies in biological solutions. The rate of this solidification can be easily controlled by the degree of substitution of hydroxyethylcellulose with silicoalkoxy groups.
Keywords: Biocompatible Materials; chemistry; Bone and Bones; surgery; Cellulose; analogs & derivatives; chemistry; Composite Resins; chemistry; Humans; Hydrogen-Ion Concentration; Materials Testing; Silicon; analysis; chemistry; Surgery, Oral; Temperature
Introduction
Contemporary approaches to the reconstruction of bone tissue in orthopaedic surgery, stomatology and dental applications rely on calcium-phosphate ceramics. Calcium phosphate (CaP) biomaterials are used in bone repair, substitution or augmentation and as osteoconductive fillers to achieve bone coalescence [1–4]. CaP are the principal raw materials used for bone substitutes in the elaboration of granules or blocks [2]. The macro porosity of calcium phosphate blocks facilitates the penetration of cells and biological growth factors in the implant. The osteogenesis process may occur in these cases within the inner surface of the pores. However, these forms are of limited value when cavities are not easily accessible, which is the case in most dental applications and in orthopaedics when percutaneous surgery is required. Injectable biomaterial has recently been developed [5], which combines a viscous phase consisting of a polymeric water solution (non-ionic cellulose ether) and a bioactive calcium phosphate (CaP) ceramic filler [6]. The fillers are maintained in viscous phase at the surgical site and the hydrophilic polymer maintains space between the ceramic granules to facilitate the development of growth factors and cells inside the material. This formulation can be modified to provide ready-to-use sterilised injectable material that decreases the risk of infection since the product does not need to be prepared during surgery.
The principal disadvantage of this type of injectable bone substitute (IBS) is its tendency to displace (to move away from) in place where it was implanted because of its liquid state. The implant need to resist all strength on the surgical site before bone substitution occurs, Such strengths may cause micro-movements of the implant constituents prevents the cell form the synthesis of the bone. As a result the initial shape of the implant may be lost.
In order to eliminate these drawbacks several liquid type suspensions or pastes, which harden in situ in the place of their application, have been tested. Among others so called ionic cements belong to this group. The hardening of these materials is caused by the crystallisation of a mineral phase which is different of the starting materials [7]. For example Norian ® [8] is new ionic cement that hardens in under physiological conditions. They consist of blend of Monocalcium phosphate, monohydrate (MCPM) with α-tricalcium phosphate (TCP) and calcium carbonate (CC). When a sodium phosphate solution is added and mixed with the powder the hardening reaction occur. The main drawback of these systems is the necessity of the mixing of the appropriate component prior to their use. The other disadvantage is their relative dense structure after hardening, which is not quickly colonised by cells.
The described above disadvantage has been eliminated in the case of a new composite presented in this communication [9]. Our main goal was to prepare ,,a ready for use” liquid type paste whose rheological properties do not change upon extending storage time. This paste consists of a mineral phase (biphasic calcium phosphate) suspended in a modified hydroxyethylcellulose solution. Sau and Majewicz [10,11] demonstrated that the functionalisation of cellulose derivatives or other polysaccharides, with silicoalkoxy substituents containing alkoxygroups significantly changes the rheological properties of these polymers. In aqueous media they either dissolve or undergo gellation depending of the pH of the medium [10,11]. In our new composite materials we used hydroxyethylcellulose as the mineral phase binder. The polymer was previously modified via the reaction with 3-glycidoxypropyltrimethoxysilane. The polymer has already been positively tested in vitro for toxic and mutagenic action of selected cells and bacteria (ISO/TR/7405/84) [12]
Experimental
For the preparation of the modified hydroxyethylcellulose polymers (HEC) commercially available compounds were used (Aqualon, Rueil-Malmaison, France), namely: Natrosol 250 G (HEC L), Natrosol 250 M (HEC M) and Natrosol 250 HH (HEC H). The modification process has been carried out in an inert gas atmosphere. A typical preparation can be described as follows: a suspension was prepared by mixing 20 g of the polymer with 500 ml of 1-propanol which was then deoxygenated for 30 min in a flow of nitrogen. Then 1 g of sodium hydroxide was introduced to the reaction mixture and finally an appropriate amount of 3-glycidoxypropyltrimethoxysilane (SIL) (Aldrich) was added dropwise. The reaction was carried out for 5 h at the temperature of the boiling point of the solvent. Then the reaction mixture was cooled down and 5 ml of anhydrous acetic acid was added. The polymer was separated by filtration, washed repeatedly with acetone and finally dried at 50 °C for 1h. The reaction scheme is presented in Fig. 1.
Fig. 1.
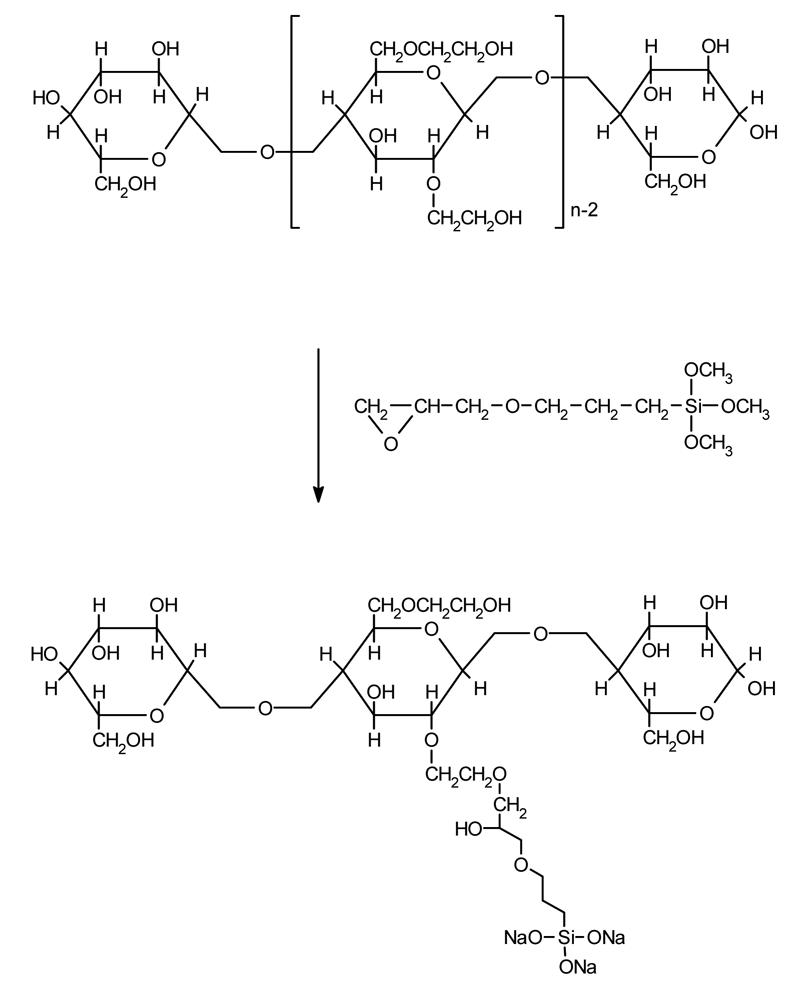
Hydroxyethylcellulose modification via addition with 3-glycidoxypropyltrimethoxysilane
The degree of substitution was estimated by analytical determination of Si. (Atomic absorption spectroscopy; Unicam 989, UK).
Apparent viscosity measurements were done to control kinetic and pH dependence of the self-hardening using a Visco Star-L, Fungilab, viscometer (T = 25 °C, speed of rotation 2 rpm, polymer concentration 1 wt %). The pH (pH meter Knick) was decreased with drops of concentrated HCl.
We have also tested the behaviour of the suspension consisting of 2 wt % solution of the modified polymer (pH 11–12) and biphasic calcium phosphate ceramic (BCP; 60% hydroxyapatite and 40% β-tricalcium phosphate). Two weight ratios of the polymer solution to BCP were selected 40:60 and 60:40. To control the self-hardening of the blend, a Brookfield® viscometer RDV1+ (T = 25°C, spindle N° 27–29; 1 rpm) was used. The composite suspension was put in a filter (Thimble/Filter 16×50 mm, Schleiderand schmell). The full filter was put into constant pH buffer (Phosphate Buffer Solution – PBS, Dulbecco Seromed, France) The torque measurements of the Brookfield® viscometer were recorded with a computer.
Thermogravimetric mesurements was performed with a DSC 2010, TA Instruments.
Results and discussion
The silicon content results obtained for selected polymers are collected in Table I. On the basis of these results it can be concluded that the yield of the addition varied from 45–90 % depending on the type of hydroxyethylcellulose used and of the 3-glycidoxypropyltrimethoxysilane to hydroxyethylcellulose ratio. Better yields are obtained for low molecular weight hydroxyethylcellulose and for higher silane to polymer ratio. The modified polymers unlike unmodified hydroxyethylcellulose do not dissolve in neutral pH aqueous solution. However the increase of pH to 11–12 leads to a complete dissolution of the polymer. Upon consecutive lowering of pH gellation process occurs. This gellation can be conveniently monitored by the determination of the viscosity of the solution as a function of pH of the solution. Such relationship is presented in Fig 2. Upon titration of the polymer solution with an acid a quick drop of pH occurs without a significant change of the viscosity. Further addition of the acid provokes gellation, which is manifested by an abrupt increase of the viscosity. The onset of the gellation depends on the amount of sillyl groups in the modified polymer. In general it is shifted to higher values of pH with increasing silicon content. Molecular weight has similar effect on the gellation. For higher molecular weight hydroxyethylcellulose (HEC-SIL H) the gellation is observed for higher pH values.
Table 1.
Analytically determined silicon content in modified hydroxyethylcellulose polymers.
| Polymer synthesis | Silicon content in polymer [%] | Yield of addition [%] |
|---|---|---|
| Natrosol 250HH + 1 ml SIL: HEC-SIL H1 | 0.55 | 90.3 |
| Natrosol 250HH + 5 ml SIL: HEC-SIL H5 | 1.70 | 67.7 |
| Natrosol 250G + 1.5 ml SIL: HEC-SIL L1.5 | 0.40 | 45.5 |
| Natrosol 250G + 2 ml SIL: HEC-SIL L2 | 0.55 | 47.8 |
| Natrosol 250G + 4 ml SIL: HEC-SIL L4 | 1.25 | 59.5 |
Fig. 2.
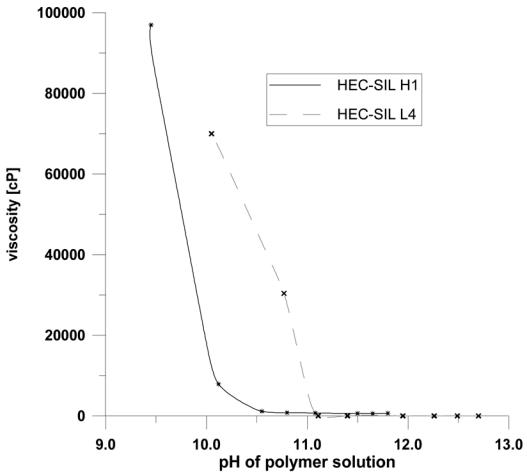
Viscosity dependence on pH for selected modified hydroxyethylcellulose polymers. The studies were carried out on a visco-star viscosimeter. The parameters of the measurement were as follows: T = 25 °C, speed of rotation 2 rev./min, polymer concentration 1 wt %.
Thermal stability of the modified polymers was tested by thermogravimetry. The TG curves are presented in Fig. 3. Unmodified hydroxyethylcellulose decomposes above 350 °C. The modification with sillyl groups has a negative effect on the thermal stability of the polymer. The onset of the thermal degradation is in this case downshifted to ca 250 °C. This value is however sufficiently high to hope that thermal sterilisation of the composite occurs without the deterioration of its molecular weight and properties.
Fig. 3. Thermogravimetric curves for selected modified hydroxyethylcellulose polymers.
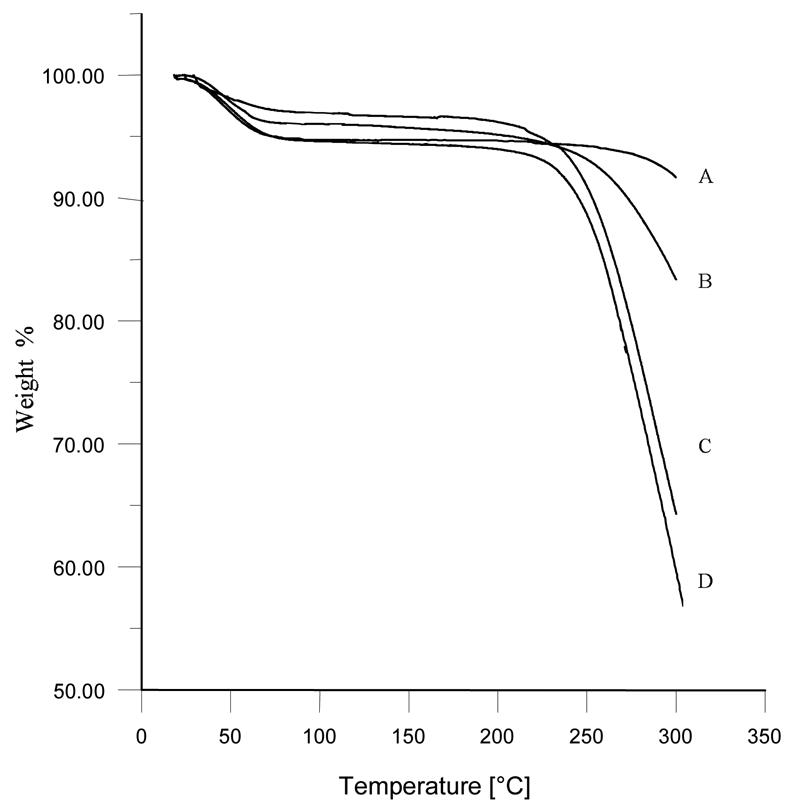
A: HEC H
B: HEC-SIL H5
C: HEC-SIL L4
D: HEC-SIL H1
To control the self-hardening of the composite in a biological buffer, Brookfield® torque variation (linked to apparent viscosity) vs. time of this immersion for selected modified hydroxyethylcellulose - BCP composite is presented in Fig. 4. A constant increase of viscosity was observed in all cases whose rate dependent on the degree of polymer modification. In conclusion it can be stated that the properties of hydroxyethylcellulose can be significantly altered by the introduction of a small amount of silicoalkoxy groups.
Fig. 4. Viscosity vs. time of immersion in PBS buffer measured for selected HEC-SIL/BCP composite.
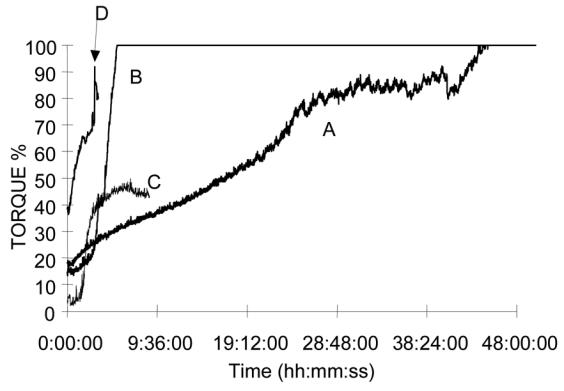
A: HEC-SIL H1 2% solution 60% / BCP40%
B: HEC-SIL L1.5 2% solution 60% / BCP 40%
C: HEC-SIL L4 2% solution 60% / BCP 40%
D: HEC-SIL H5 2% solution 60% / BCP 40%
Conclusions
To summarise, we have elaborated a new biomaterial with easily controllable, pH dependent rheological properties. This HEC-SIL/BCP composite exhibits good thermal stability up to 250 °C, which enables its thermal sterilisation. This new approach in the fabrication of “in situ” hardening biomaterials opens up the possibility of the preparation of a whose series of injectable biocomposites with strictly defined properties, without the necessity of the addition of reticulation agent, generally cytotoxic.
References
- 1.Jarcho M. Clin Orthopaed. 1981;157:257. [PubMed] [Google Scholar]
- 2.De Groot K. Bioceramics of Calcium Phosphate. CRC Press; Boca Raton, FL: 1983. [Google Scholar]
- 3.Nery B, LeGeros RZ, Lynch K, Lee K. J Periodont. 1992;63:729. doi: 10.1902/jop.1992.63.9.729. [DOI] [PubMed] [Google Scholar]
- 4.LeGeros RZ, Daculsi G. In: Handbook of Bioactive Ceramics, Calcium Phosphate and Hydroxyapatite Ceramics. Yamamuro T, Hench LL, Wilson J, editors. CRC Press; Boca Raton, FL: 1990. pp. 17–28. [Google Scholar]
- 5.Daculsi G, Weiss P, Delecrin J, Grimandi G, Passuti N, Guerin F. WO 95/21634 Int Pat.
- 6.Daculsi G. Biomaterials. 1998;191:473. doi: 10.1016/s0142-9612(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 7.Lemaître J. Inov Tech Biol Med. 1995;16:109. [Google Scholar]
- 8.Constantz BR, Ison IC, Fulmer MT, Poser RD, Smith ST, VanWagoner M, Ross J, Goldstein SA, Jupiter JB, Rosenthal DI. Science. 1995;267:1796. doi: 10.1126/science.7892603. [DOI] [PubMed] [Google Scholar]
- 9.Lapkowski M, Weiss P, Daculsi G, Dupraz A. WO 97/05911 Int Pat.
- 10.Sau AC. Polymer Preprints. 1990;31:636. [Google Scholar]
- 11.Sau AC, Majewicz TG. ACS Symp Ser. 1992;476:265. [Google Scholar]
- 12.Wierucka-Mlynarczyk B. Ph D thesis. Silesian Medical University; Zabrze: 1998. [Google Scholar]


