Abstract
Dopamine (DA) in the medial preoptic area (MPOA) provides important facilitative influence on male rat copulation. We have shown that the nitric oxide-cGMP (NO-cGMP) pathway modulates MPOA DA levels and copulation. We have also shown that systemic estradiol (E2) maintains neuronal NO synthase (nNOS) immunoreactivity in the MPOA of castrates, as well as relatively normal DA levels. This effect of E2 on nNOS probably accounts for at least some of the previously demonstrated behavioral facilitation by intra-MPOA E2 administration in castrates. Therefore, we hypothesized that stimulation of the MPOA NO-cGMP pathway in dihydrotestosterone (DHT)-treated castrates should restore DA levels and copulatory behaviors. Reverse-dialysis of a NO donor, sodium nitroprusside (SNP), increased extracellular DA in the MPOA of DHT-treated castrates and restored the ability to copulate to ejaculation in half of the animals. A cGMP analog, 8-Br-cGMP, also increased extracellular DA, though not as robustly, but did not restore copulatory ability. The effectiveness of the NO donor in restoring copulation and MPOA DA levels is consistent with our hypothesis. However, the lack of behavioral effects of 8-Br-cGMP, despite its increase in MPOA DA, suggests that NO may have additional mediators in the MPOA in the regulation of copulation. Furthermore, the suboptimal copulation seen in the NO donor-treated animals suggests the importance of extra-MPOA systems in the regulation of copulation.
Keywords: male sexual behavior, nitric oxide, dopamine, cGMP, MPOA, DHT
Gonadal steroids exert strong influence on copulation in male rats (see Hull, Wood, and McKenna, 2006 for review). Testosterone (T), the major gonadal steroid in male rats, can be converted in various tissues to its androgenic metabolite dihydrotestosterone (DHT) or its estrogenic metabolite 17β-estradiol (E2). Both metabolites are required for full expression of male copulation (Baum and Vreeburg, 1973; Feder, Naftolin, and Ryan, 1974; Larsson, Södersten, and Beyer, 1973). A target of E2 is the medial preoptic area (MPOA), one of the most important structures for the regulation of male copulation. Davis and Barfield (1979) demonstrated that implantation of E2 in this area, along with systemic administration of DHT, restored and maintained copulation in castrates, while MPOA E2 or systemic DHT alone was not as effective. The importance of E2 in the MPOA for copulation was further supported by more recent studies using an aromatase inhibior (Clancy, Zumpe, and Michael, 1995; Clancy, Zumpe, and Michael, 2000). Thus, E2 in the MPOA contributes to the restoration and maintenance of copulation in castrates. Androgenic stimulation of the MPOA also contributes to the full expression of male sexual behaviors, through its effects on sexual motivation and copulation (Harding and McGinnis, 2004; McGinnis, Montana, and Lumia, 2002). Systemic DHT maintains genital structures and sensory and motor processing neurons as well (reviewed in Hull et al., 2006).
Recently, we have identified neuronal nitric oxide synthase (nNOS) as a possible target for the estrogens in the MPOA. NOS inhibitors microinjected into the MPOA inhibit copulation (Lagoda, Muschamp, Vigdorchik, and Hull, 2004; Sato, Horita, Kurohata, Adachi, and Tsukamoto, 1998), and NO facilitates basal and female-stimulated DA release in the MPOA (Lorrain and Hull, 1993; Lorrain, Matuszewich, Howard, Du, and Hull, 1996), mediated by the NO-cGMP pathway (Sato and Hull, 2006). Furthermore, MPOA nNOS neurons may contain androgen receptor (AR) and/or estrogen receptor α (ERα) in rats (Sato, Braham, Putnam, and Hull, 2005) and mice (Scordalakes, Shetty, and Rissman, 2002). nNOS-immunoreactivity (ir) is reduced in castrates (Du and Hull, 1999), while systemic T or E2 maintains nNOS-ir (Du and Hull, 1999; Putnam, Sato, Riolo, and Hull, 2005). This pattern closely matches the MPOA extracellular DA levels, an important predictor of copulatory performance (reviewed in Hull, Muschamp, and Sato, 2004). In castrates, extracellular DA levels are reduced, while intracellular DA levels are increased, presumably due to impaired release (Du, Lorrain, and Hull, 1998). Such changes can be attenuated by systemic administration of T or E2, while systemic DHT may also contribute to a lesser degree (Putnam, Sato, and Hull, 2003; Putnam et al., 2005; Sato, Shibuya, Adachi, Kato, Horita, and Tsukamoto, 1998). Taken together, the lack of estrogenic stimulation of the MPOA (from T or E2) seems to impair nNOS function, reducing NO production. This, in turn, reduces MPOA DA levels and impairs copulation.
If MPOA nNOS is the major mediator of the effects of estrogens, stimulation of the NO-cGMP pathway should facilitate MPOA DA release and copulation in DHT-treated castrates. Indeed, reverse-dialysis of either a NO donor or cGMP analog in the MPOA of gonadally intact males increased both extracellular DA and copulatory ability (Sato and Hull, 2006). We hypothesized that administration of a NO donor or a cGMP analog in the MPOA should mimic the facilitative effects of E2 seen in the study by Davis and Barfield (1979). In the current study, the effects of stimulation of the NO-cGMP pathway on MPOA DA levels and copulation were examined in DHT-treated one-month castrates.
Methods
Subjects
Adult male Long-Evans Blue Spruce rats (250−300 g), purchased from Harlan (Indianapolis, IN), were individually housed in a temperature- and humidity-controlled colony room. Food and water were available ad libitum. The colony room was maintained on a 14:10 reversed light:dark cycle with lights off at 11:00 h. All procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the local Institutional Animal Care and Use Committee (IACUC).
All animals were screened for copulatory ability 1 week after arrival, using females of the same strain that were ovariectomized and hormone-primed [10 μg estradiol benzoate and 400 μg progesterone (sc, in olive oil vehicle, Sigma-Aldrich, St. Louis, MO), 48 hrs and 4 hrs, respectively, prior to testing]. Only the animals that achieved at least 3 ejaculations during a maximum of four 30-min testing sessions were included.
Intracranial guide cannula implantation and hormone replacement
Animals were anesthetized with an intraperitoneal injection (i.p.) of ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (4 mg/kg) and castrated as previously described (Putnam, Du, Sato, and Hull, 2001). Silastic tubing (1.6 mm ID, 3.2 mm OD; Dow Corning) containing 60 mm (2 × 30 mm) of DHT were implanted, as previously described (Meisel, O'Hanlon, and Sachs, 1984).
Following castration and Silastic tubing implantation, animals were placed on a Kopf stereotaxic frame with the incisor bar set at +5 mm. Rats were implanted with a 15 mm 23 ga thin-wall stainless steel guide cannula, ending 2 mm above the left MPOA (mm from bregma: AP, +2.3; ML, 0.4; DV, −6.3; Pellegrino, Pellegrino, and Cushman, 1979). The guide cannula and a metal male electrical clip, for connecting the animal to the microdialysis setup, were secured to the top of the skull with dental cement. An obturator made from 27 ga stainless steel tubing and ending flush with the end of the guide cannula was inserted into the guide cannula to prevent debris from entering the cannula.
Drugs.
A cGMP analog, 8-Br-cGMP, and a NO donor, sodium nitroprusside (SNP), were obtained from Sigma-Aldrich (St. Louis, MO). Both drugs were previously tested on the HPLC to ensure that they did not interfere with the transmitter analysis. The doses for each drug were determined from previous studies (Sato and Hull, 2006; West and Galloway, 1998).
In vivo microdialysis
Probe construction
Microdialysis probes with a concentric flow design were constructed in house as previously described (Renno, Mullet, Williams, and Beitz, 1998). The finished probe had a 1 mm long active dialyzing membrane (MW cut off 13,000 kD, 210 μm o.d., Spectrum Laboratories, Rancho Dominguez, CA).
Microdialysis setup
On the day of testing, subjects were lightly anesthetized with ketamine hydrochloride (12.5 mg/kg) and xylazine hydrochloride (1 mg/kg), and a microdialysis probe was inserted into the guide cannula. Flow of the artificial cerebrospinal fluid (aCSF; Dulbecco's solution, Sigma-Aldrich, St. Louis, MO) began immediately upon insertion of the probe. aCSF was delivered to the microdialysis probe with a Harvard syringe pump (PHD2000, Holliston, MA) through a dual-channel swivel (Instech Laboratories, Plymouth Meeting, PA). Samples were collected into a sample vial attached to the tether line through a fused silica capillary. Following a minimum of 3 hrs stabilization period, samples were collected every 6 min, immediately frozen in powdered dry ice, and stored in a –80° C ultracold freezer until analysis. Deoxygenated aCSF was used throughout the experiment to prevent degradation of SNP.
Experimental Design
The effects of 8-Br-cGMP and SNP on MPOA DA and metabolites and copulation were examined in DHT-treated male rats one month after castration. After a 3-hr stabilization period, 3 baseline (BL) samples were collected. The last BL sample was expressed as percent of the mean of the 3 BL samples. Following BL sample collection, aCSF was switched to one containing 50 μM 8-Br-cGMP (N = 5) or 500 μM SNP (N = 6). Control animals (N=6) received regular aCSF throughout the test. Additional samples were collected for 18 min (3 samples, Drug), after which an estrous female was then introduced behind a barrier for 18 min (3 samples, EST). Subsequently, the barrier was removed and the male was allowed to copulate for 30 min (5 samples, COP), during which copulatory behaviors were recorded. Upon the conclusion of dialysis testing, animals were deeply anesthetized with Na+ pentobarbitol (50 mg/ml) and euthanized. The brains were removed and the placement of probes histologically verified under a projection microscope.
HPLC
Monoamines and their metabolites were assayed with high performance liquid chromatography with electrochemical detection (HPLC-EC). Mobile phase (pH. 3.8), consisting of 30 mM citric acid, 50 mM Na+ acetate, 0.027 mM Na+2 EDTA, 0.5 mM octane sulfonic acid (OSA), 2.5% (v/v) acetonitrile, and 0.2 % (v/v) tetrahydrofuran (THF), were delivered by a Gilson model 307 pump with a 5 ml piston head at 0.5 ml/min. The pump was equipped with a Valco flow splitter to deliver 8 μl/min to an LC-Packings Fusica II reverse-phase capillary column (300 μm I.D. and 25 mm in length, packed with Hypersil C18 BDS, Sunnyvale, CA). Samples were loaded via a Rheodyne 7520 manual injector equipped with a 0.5 μl sample loop. Electrochemically active compounds were detected with an Antec microcell (VT-03 EC flowcell with a 25 μm spacer) with a glassy carbon working electrode maintained at a potential of +0.7 V relative to a Ag/AgCl reference electrode on the Antec Decade.
Daily injections of 1 pg/μl of external standards (0.5 pg on column) were used to verify sensitivity, reproducibility, and the retention time. Using Gilson Unipoint program, the amount of DA in a sample was measured as the area under the curve of the appropriate peak in the chromatogram. The changes in DA levels were expressed as percent change relative to the average of the last 3 baseline samples (BL).
Behavioral measures
The following behavioral measures were recorded during 30 min copulation testing sessions: mount frequency during the first copulatory series (MF1), total mount frequency (MFT), intromission frequency during the first copulatory series (IF1), total intromission frequency (IFT), total ejaculation frequency (EF). In addition, latency to the first mount (ML) and first intromission (IL) following introduction of the female, latency to the first ejaculation (EL1) following the first intromission, and the duration of the postejaculatory interval following the first ejaculation (PEI1, latency from ejaculation to the next intromission) were recorded. When available, mount frequency, intromission frequency, ejaculation latency, and PEI for the subsequent copulatory series were recorded as well.
Statistical Analysis
The percent changes from the baseline were analyzed with a 2-way mixed ANOVA (drug × sample period), followed by a 1-way repeated measures ANOVA and planned pair-wise comparisons with BL, using paired sample t-tests with Bonferroni's procedure.
The proportions of animals exhibiting mount, intromission, or ejaculation were analyzed with X2 tests. For all statistical analyses, P < 0.05 was considered statistically significant.
Results
Behavioral measures
The behavioral data are shown in Table 1. More SNP-treated animals showed mounts [X2 (2) = 6.56, P = 0.038], intromissions [X2 (2) = 6.68, P = 0.035], and ejaculation [X2 (2) = 6.68, P = 0.035], than either control or 8-Br-cGMP-treated animals. Half of the SNP-treated animals ejaculated, while only one control animal mounted and no 8-Br-cGMP-treated animal showed any copulatory behaviors.
Table 1.
The effects of reverse-dialysis of a NO-donor (SNP) or a cGMP analog (8-Br-cGMP) on copulatory measures.
| Treatment | n (mounted/total) | ML | MF1 | MFT | |||
| Control | 1/6 | 232.00 | - | - | 0.33 | ± 0.33 | |
| 8-Br-cGMP | 0/5 | - | - | - | 0.00 | - | |
| SNP | 4/6 * | 14.75 | ± 2.75 | 6.00 | ± 0.58 | 5.17 | ± 2.26 |
| Treatment | n (intromitted/total) | IL | IF1 | IFT | |||
| Control | 0/6 | - | - | - | - | 0.00 | - |
| 8-Br-cGMP | 0/5 | - | - | - | - | 0.00 | - |
| SNP | 3/6 * | 150.00 | ± 103.36 | 4.67 | ± 4.67 | 3.33 | ± 1.63 |
| Treatment | n (ejaculated/total) | EL | EF | PEI | |||
| Control | 0/6 | - | - | 0.00 | - | - | - |
| 8-Br-cGMP | 0/5 | - | - | 0.00 | - | - | - |
| SNP | 3/6 * | 333.67 | ± 37.64 | 0.67 | ± 0.33 | 1022.00 | ± 788.45 |
Significantly different from control (P < 0.05). ML, mount latency; MF1, mount frequency in the first ejaculatory series; MFT, total mount frequency; IL, intromission latency; IF1, intromission frequency in the first ejaculatory series; IFT, total intromission frequency; EL, ejaculation latency; EF ejaculation frequency; PEI, postejaculatory interval before the next intromission.
DA and metabolites levels
A 2-way ANOVA on DA levels revealed a significant main effect of sample period [F(11, 154) = 7.39, P < 0.0001], a main effect of drug-treatment [F(2, 154) = 288.57, P < 0.0001], and an interaction [F(22, 154) = 2.51, P = 0.001]. Follow-up 1-way ANOVAs on each group revealed significant changes in extracellular DA levels in 8-Br-cGMP-treated [F(11, 44) = 5.36, P < 0.0001] and SNP-treated [F(11, 55) = 4.41, P < 0.0001] males, while no DA level change was detected in control animals [F(11, 55) = 1.02, NS]. In SNP-treated animals, DA levels significantly increased relative to baseline in all samples during which the drug was administered. In 8-Br-cGMP-treated animals, DA levels significantly increased relative to baseline, reaching significance at the second and the third estrous female samples (EST-2 and EST-3), as well as at the first and the last copulation samples (COP-1 and COP-5, Fig. 1a).
Fig. 1.
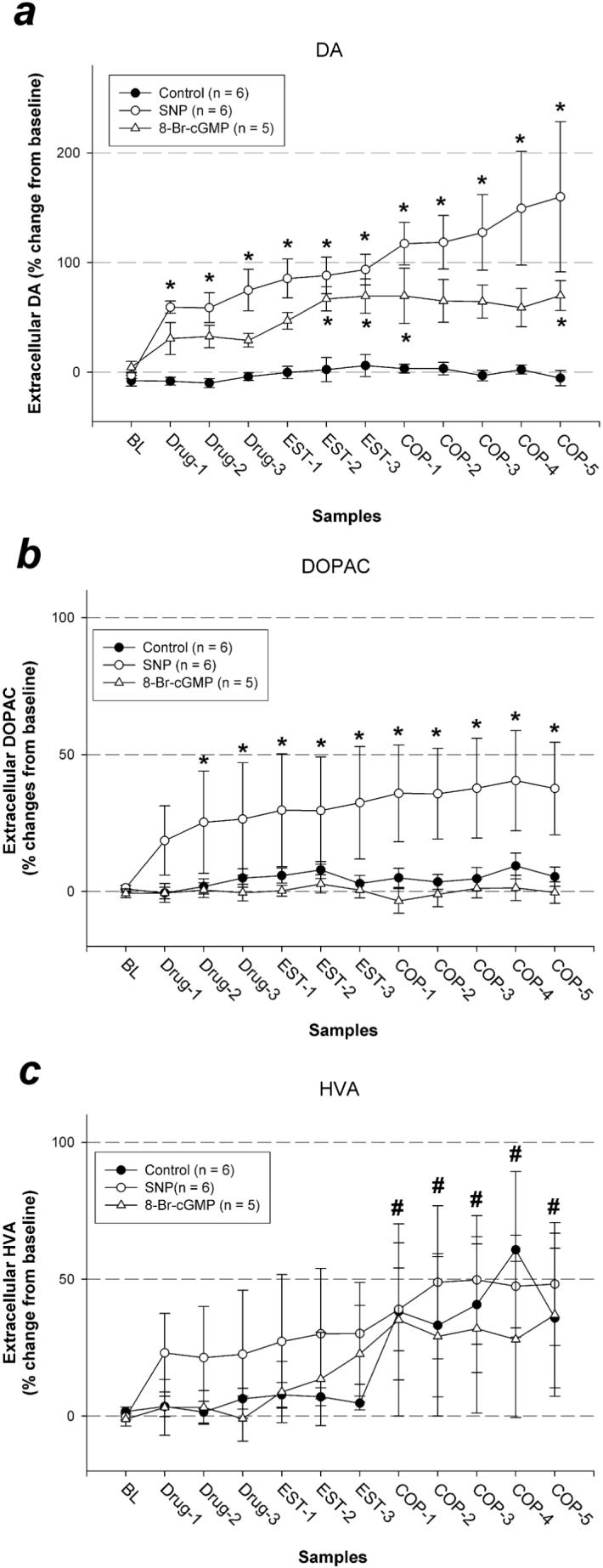
Percent changes in MPOA DA (a), DOPAC (b) and HVA (c) levels during baseline (BL), drug pre-treatment (Drug), exposure to an estrous female behind a barrier (EST), and copulation testing (COP). * Significantly different from average baseline (P < 0.05). # Overall average significantly different from overall average baseline (P < 0.05).
A 2-way ANOVA on DOPAC revealed a significant main effect of sample period [F(11, 154) = 2.74, P = 0.003], a main effect of drug-treatment [F(2, 154) = 353.01, P < 0.0001], and an interaction [F(22, 154) = 1.67, P = 0.038]. Follow-up 1-way ANOVAs on each group revealed significant changes in DOPAC levels in SNP-treated animals [F(11, 55) = 2.69, P = 0.008], while no change was detected in control [F(11, 55) = 1.01, NS] or 8-Br-cGMP-treated animals [F(11, 44) = 0.38, NS]. In SNP-treated animals, DOPAC levels increased relative to baseline in all but the first sample during which drug was administered (Fig. 1b).
A 2-way ANOVA on HVA revealed a significant main effect of sample period [F(11, 154) = 5.27, P < 0.0001], while the main effect of drug-treatment [F(2, 154) = 0.24, NS] and the interaction [F(22, 154) = 0.39, NS] were not significant. On average, HVA levels were higher during copulation testing compared to baseline regardless of the drug treatment (Fig. 1c).
Discussion
As expected, both SNP and 8-Br-cGMP increased MPOA DA levels in DHT-treated one-month castrates. However, their differential effects on copulatory behaviors were somewhat unexpected. SNP-treated animals exhibited behaviors comparable to those treated with MPOA E2 and systemic DHT in the study by Davis and Barfield (1979), while 8-Br-cGMP-treated animals showed virtually no copulatory behavior, despite the significant increase in MPOA DA levels with this treatment.
To our knowledge, this is the first report of a dissociation between MPOA extracellular DA levels and copulatory performance. Several factors may have contributed to the present discrepancy. First, it should be noted that the basal extracellular DA levels are reduced in the MPOA of oil- or DHT-treated castrates, compared to E2- or T-treated castrates or gonadally intact animals (Du and Hull, 1999; Putnam et al., 2005). Therefore, the relatively small increase in DA levels with 8-Br-cGMP may not have been enough to reach threshold for activation of copulation. Second, NO may facilitate copulation through multiple pathways. SNP, a NO donor, was effective in restoring copulation and increasing DA levels, while the cGMP analog was not effective in restoring copulation, and its effects on DA were relatively smaller than expected from the data in gonadally intact animals (Sato and Hull, 2006). This indicates that the lack of E2 affects not only nNOS, but also an effector for the cGMP. Perhaps the effects of NO are mediated through additional pathways that are not affected by lack of E2. Other mediators of NO's effects include S-nitrosylation (reviewed in Ahern, Klyachko, and Jackson, 2002) or ADP-ribosylation (reviewed in Schlossmann, Feil, and Hofmann, 2003) of synaptic proteins. It is possible that NO can facilitate copulation through such mechanisms, independent of the NO-cGMP pathway. Finally, the volume of tissue affected by the reverse-dialysis is relatively small, which may not have been sufficient to influence behavior. In most of the previous studies showing a very close relationship between extracellular DA and copulatory ability (Dominguez, Riolo, Xu, and Hull, 2001; Hull, Du, Lorrain, and Matuszewich, 1995; Putnam et al., 2001; Putnam et al., 2003; Sato and Hull, 2006), endogenous processes increased DA release, presumably throughout the MPOA, before and during copulation.
In addition to its effects on DA release and sexual behavior, the MPOA NO-cGMP pathway is implicated in regulation of GnRH secretion in male (Pu, Kalra, and Kalra, 1998) and female (Pu, Xu, Kalra, and Kalra, 1996) rats. There are inconsistent reports of facilitation of sexual behavior by GnRH (reviewed in Hull et al., 2006). However, it seems unlikely that GnRH is the “other” mediator of NO's effects in these experiments, since cGMP was the mediator of the NO-induced GnRH release in the earlier experiments, and 8-Br-cGMP did not facilitate sexual behavior in this experiment.
Copulation in castrates is not completely restored, either with systemic DHT and intra-MPOA E2 (Davis and Barfield, 1979) or with systemic DHT and intra-MPOA NO in this study. This suggests that estrogenic influence in structures outside the MPOA is also required for full restoration of copulatory behaviors. It appears that the extra-MPOA DA plays an important role in this regard. In the presence of circulating androgens, systemic apomorphine (APO, a classic D1/D2 DA agonist) was sufficient to stimulate normal copulation in T-treated ERα-knock out (ERαKO) mice, which normally show little copulatory behavior (Wersinger and Rissman, 2000). However, microinjection of APO icv, which likely had limited and uneven diffusion in the brain, only partially restored copulation in ERαKO mice (Scordalakes and Rissman, 2003). Furthermore, either systemic or intra-MPOA administration of APO was only partially effective in restoring copulation in long-term castrates in the absence of circulating androgens or estrogens (Scaletta and Hull, 1990). One of the structures where E2 should have a significant influence is the medial amygdala, where E2 implants were effective in restoring copulation in castrated hamsters (Wood, 1996). Finally, estrogenic influence in the MPOA independent of NO may be required, given the large number of ER-positive cells in the MPOA without nNOS (Sato, et al., 2005; Scordalakes, et al., 2002). Taken together, circulating androgens and intra- and extra-MPOA DA all seem to be required for the expression of normal copulation.
In conclusion, our initial hypothesis that E2 in the MPOA stimulates copulation through the NO-cGMP-DA pathway was partially supported. The effectiveness of a NO donor in restoring copulation and MPOA DA levels is consistent with our model. However, the lack of behavioral effects of 8-Br-cGMP, despite its increase in MPOA DA, suggests that NO may have additional mediators in the MPOA in the regulation of copulation. Furthermore, the suboptimal copulation seen in the NO donor-treated animals suggests the importance of extra-MPOA DA systems in the regulation of copulation.
Acknowledgements:
This research was supported by NIH grants R01 40826 and K02 01714 to EMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25:510–517. doi: 10.1016/s0166-2236(02)02254-3. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Vreeburg JT. Copulation in castrated male rats following combined treatment with estradiol and dihydrotestosterone. Science. 1973;182:283–5. doi: 10.1126/science.182.4109.283. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Zumpe D, Michael RP. Intracerebral infusion of an aromatase inhibitor, sexual behavior and brain estrogen receptor-like immunoreactivity in intact male rats. Neuroendocrinology. 1995;61:98–111. doi: 10.1159/000126830. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Zumpe D, Michael RP. Estrogen in the medial preoptic area of male rats facilitates copulatory behavior. Horm. Behav. 2000;38:86–93. doi: 10.1006/hbeh.2000.1602. [DOI] [PubMed] [Google Scholar]
- Davis PG, Barfield RJ. Activation of masculine sexual behavior by intracranial estradiol benzoate implants in male rats. Neuroendocrinology. 1979;28:217–27. doi: 10.1159/000122865. [DOI] [PubMed] [Google Scholar]
- Dominguez J, Riolo JV, Xu Z, Hull EM. Regulation by the medial amygdala of copulation and medial preoptic dopamine release. J. Neurosci. 2001;21:349–355. doi: 10.1523/JNEUROSCI.21-01-00349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Hull EM. Effects of testosterone on neuronal nitric oxide synthase and tyrosine hydroxylase. Brain Res. 1999;836:90–98. doi: 10.1016/s0006-8993(99)01618-2. [DOI] [PubMed] [Google Scholar]
- Du J, Lorrain DS, Hull EM. Castration decreases extracellular, but increases intracellular, dopamine in medial preoptic area of male rats. Brain Res. 1998;782:11–17. doi: 10.1016/s0006-8993(97)01144-x. [DOI] [PubMed] [Google Scholar]
- Feder HH, Naftolin F, Ryan KJ. Male and female sexual responses in male rats given estradiol benzoate and 5 alpha-androstan-17 beta-ol-3-one propionate. Endocrinology. 1974;94:136–41. doi: 10.1210/endo-94-1-136. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiol. Behav. 2004;81:671–80. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J. Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol. Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Hull EM, Wood RI, McKenna KE. Neurobiology of male sexual behavior. In: Neill JD, editor. Physiology of Reproduction. Elsevier Press; New York: 2006. pp. 1729–1824. [Google Scholar]
- Lagoda GA, Muschamp JW, Vigdorchik AV, Hull EM. A NOS inhibitor in the MPOA inhibits copulation and stimulus sensitizaton in male rats. Behav. Neurosci. 2004;118:1317–23. doi: 10.1037/0735-7044.118.6.1317. [DOI] [PubMed] [Google Scholar]
- Larsson K, Sodersten P, Beyer C. Induction of male sexual behaviour by oestradiol benzoate in combination with dihydrotestosterone. J. Endocrinol. 1973;57:563–4. doi: 10.1677/joe.0.0570563. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Hull EM. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport. 1993;5:87–89. doi: 10.1097/00001756-199310000-00024. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Matuszewich L, Howard RV, Du J, Hull EM. Nitric oxide promotes medial preoptic dopamine release during male rat copulation. Neuroreport. 1996;8:31–34. doi: 10.1097/00001756-199612200-00007. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Montana RC, Lumia AR. Effects of hydroxyflutamide in the medial preoptic area or lateral septum on reproductive behaviors in male rats. Brain Res. Bull. 2002;59:227–34. doi: 10.1016/s0361-9230(02)00869-9. [DOI] [PubMed] [Google Scholar]
- Meisel RL, O'Hanlon JK, Sachs BD. Differential maintenance of penile responses and copulatory behavior by gonadal hormones in castrated male rats. Horm. Behav. 1984;18:56–64. doi: 10.1016/0018-506x(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. 2nd Plenum; New York: 1979. [Google Scholar]
- Pu S, Kalra PS, Kalra SP. Diurnal rhythm in cyclic GMP/nitric oxide efflux in the medial preoptic area of male rats. Brain Res. 1998;808:310–312. doi: 10.1016/s0006-8993(98)00821-x. [DOI] [PubMed] [Google Scholar]
- Pu S, Xu B, Kalra SP, Kalra PS. Evidence that gonadal steroids modulate nitric oxide efflux in the medial preoptic area: effects of N-methyl-D-aspartate and correlation with luteinizing hormone secretion. Endocrinology. 1996;137:1949–1955. doi: 10.1210/endo.137.5.8612535. [DOI] [PubMed] [Google Scholar]
- Putnam SK, Du J, Sato S, Hull EM. Testosterone restoration of copulatory behavior correlates with medial preoptic dopamine release in castrated male rats. Horm. Behav. 2001;39:216–224. doi: 10.1006/hbeh.2001.1648. [DOI] [PubMed] [Google Scholar]
- Putnam SK, Sato S, Hull EM. Effects of testosterone metabolites on copulation and medial preoptic dopamine release in castrated male rats. Horm. Behav. 2003;44:419–26. doi: 10.1016/j.yhbeh.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Putnam SK, Sato S, Riolo JV, Hull EM. Effects of testosterone metabolites on copulation, medial preoptic dopamine, and NOS-immunoreactivity in castrated male rats. Horm. Behav. 2005;47:513–522. doi: 10.1016/j.yhbeh.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Renno WM, Mullet MA, Williams FG, Beitz AJ. Construction of 1mm microdialysis probe for amino acids dialysis in rats. J. Neurosci. Methods. 1998;79:217–228. doi: 10.1016/s0165-0270(97)00192-1. [DOI] [PubMed] [Google Scholar]
- Sato S, Braham CS, Putnam SK, Hull EM. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: co-localization and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Res. 2005;1043:205–13. doi: 10.1016/j.brainres.2005.02.074. [DOI] [PubMed] [Google Scholar]
- Sato SM, Hull EM. The nitric oxide-guanosine 3',5'-cyclic monophosphate pathway regulates dopamine efflux in the medial preoptic area and copulation in male rats. Neuroscience. 2006;139:417–28. doi: 10.1016/j.neuroscience.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Sato Y, Horita H, Kurohata T, Adachi H, Tsukamoto T. Effect of the nitric oxide level in the medial preoptic area on male copulatory behavior in rats. Am. J. Physiol. 1998;274:R243–7. doi: 10.1152/ajpregu.1998.274.1.R243. [DOI] [PubMed] [Google Scholar]
- Sato Y, Shibuya A, Adachi H, Kato R, Horita H, Tsukamoto T. Restoration of sexual behavior and dopaminergic neurotransmission by long term exogenous testosterone replacement in aged male rats. J. Urol. 1998;160:1572–5. [PubMed] [Google Scholar]
- Scaletta LL, Hull EM. Systemic or intracranial apomorphine increases copulation in long-term castrated male rats. Pharmacol. Biochem. Behav. 1990;37:471–475. doi: 10.1016/0091-3057(90)90015-a. [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann. Med. 2003;35:21–7. doi: 10.1080/07853890310004093. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Central administration of apomorphine facilitates sexual behaviors in ERalpha KO mice. Hormones & Behavior. 2003;44:77. [Google Scholar]
- Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J. Comp. Neurol. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF. Dopamine activates masculine sexual behavior independent of the estrogen receptor alpha. J. Neurosci. 2000;20:4248–4254. doi: 10.1523/JNEUROSCI.20-11-04248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Galloway MP. Nitric oxide and potassium chloride-facilitated striatal dopamine efflux in vivo: role of calcium-dependent release mechanisms. Neurochem. Int. 1998;33:493–501. doi: 10.1016/s0197-0186(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Wood RI. Estradiol, but not dihydrotestosterone, in the medial amygdala facilitates male hamster sex behavior. Physiol. Behav. 1996;59:833–41. doi: 10.1016/0031-9384(95)02204-x. [DOI] [PubMed] [Google Scholar]


