Abstract
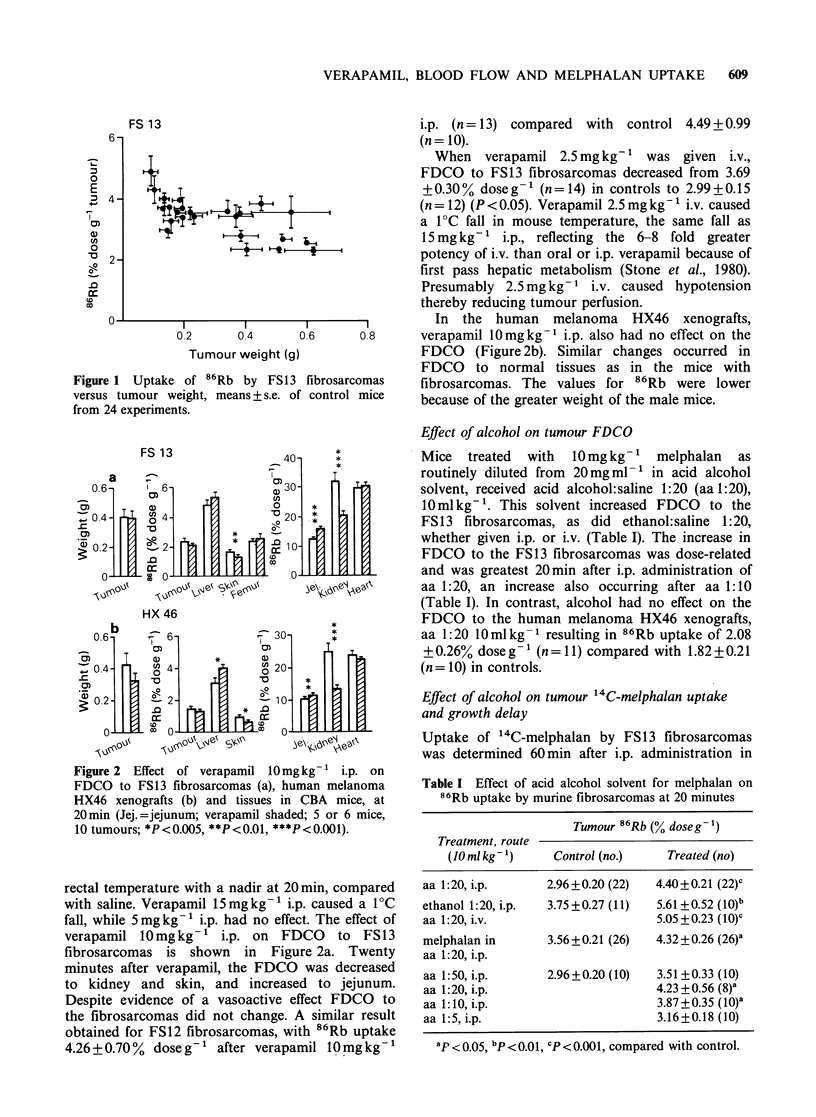
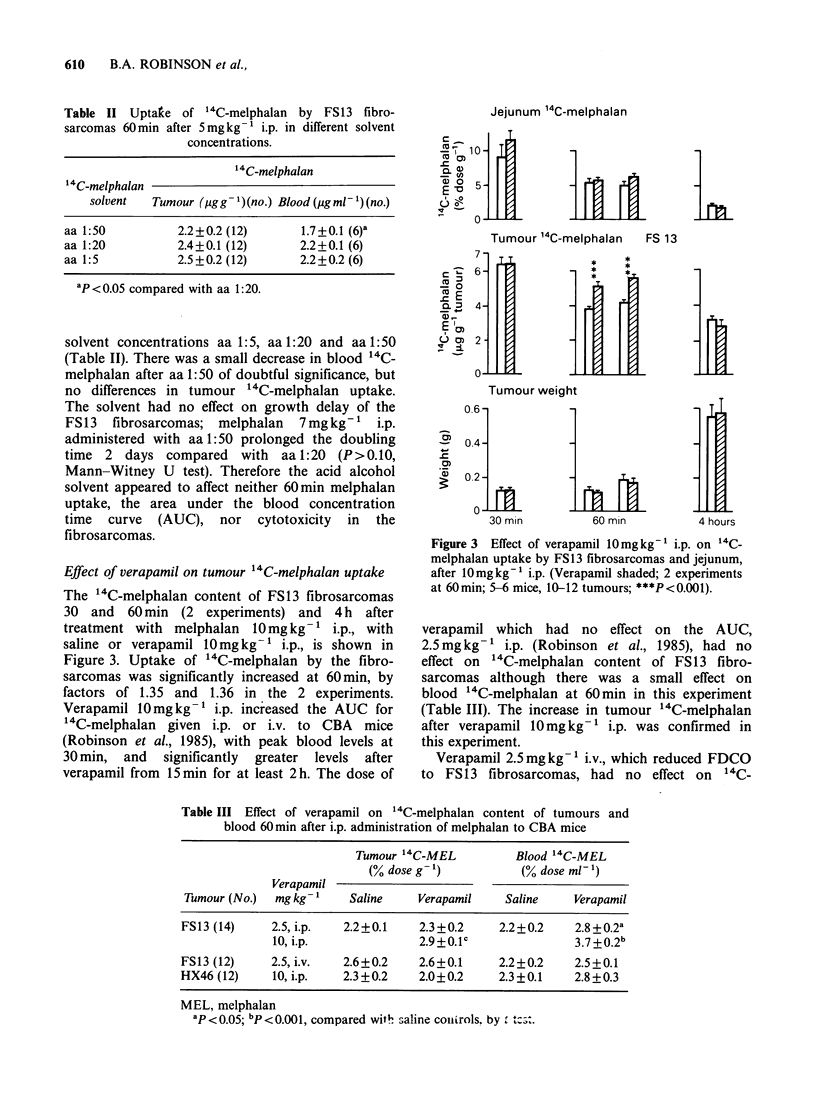
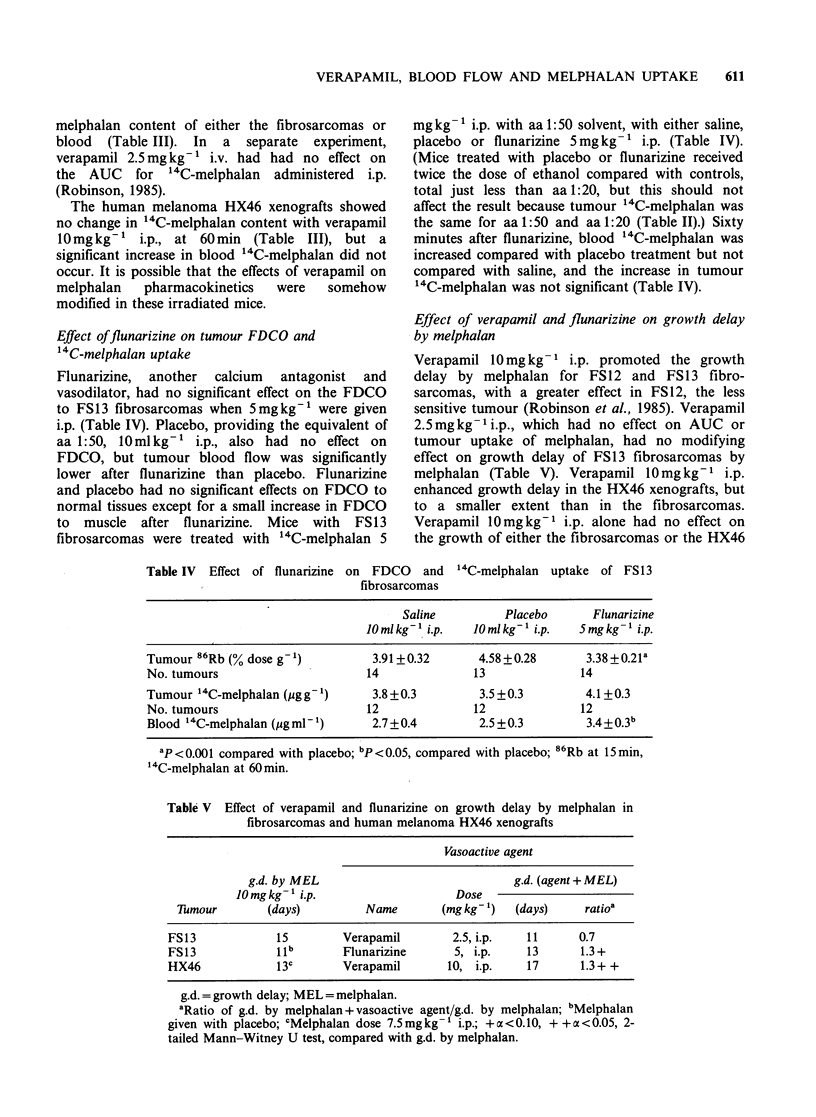
Verapamil had previously been shown to increase cellular melphalan uptake and cytotoxicity in fibrosarcomas, and increased the area under the blood concentration versus time curve (AUC) for melphalan in CBA mice. Verapamil (10 mg kg-1 i.p.) had no effect on the fractional distribution of cardiac output (FDCO), measured with 86Rb-rubidium chloride, to subcutaneous fibrosarcomas. 14C-Melphalan uptake by FS13 fibrosarcomas was increased 60 min after verapamil (10 mg kg-1 i.p.), but not after lower doses which did not affect the AUC. Flunarizine (5 mg kg-1 i.p.) also had no effect on FDCO to FS13 fibrosarcomas, and tended to increase 14C-melphalan content of blood and the fibrosarcomas and to promote growth delay by melphalan. Alcohol increased FDCO to FS13 fibrosarcomas, maximally at a 1:20 dilution in saline, but had no effect on 14C-melphalan uptake or growth delay. Thus, melphalan cytotoxicity correlated with tumour melphalan uptake, and both followed changes in the AUC for melphalan but not changes in FDCO. In these murine fibrosarcomas melphalan uptake and cytotoxicity were not limited by blood flow. In subcutaneous human melanoma HX46 xenografts, verapamil had no effect on the FDCO, nor on 14C-melphalan uptake, and did not affect blood 14C-melphalan levels, suggesting absence of effects on the AUC and on cellular uptake. Alcohol did not increase the FDCO to HX46 xenografts, providing evidence for a different vascular supply.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasberg R., Horowitz M., Strong J., Molnar P., Patlak C., Owens E., Fenstermacher J. Regional measurements of [14C]misonidazole distribution and blood flow in subcutaneous RT-9 experimental tumors. Cancer Res. 1985 Apr;45(4):1692–1701. [PubMed] [Google Scholar]
- Brown R. K., Duncan G., Hill D. L. Distribution and elimination of melphalan in rats and monkeys and distribution in tumors of mice bearing L1210 or P388 leukemias sensitive and resistant to this agent. Cancer Treat Rep. 1980 Apr-May;64(4-5):643–648. [PubMed] [Google Scholar]
- Chan R. C., Babbs C. F., Vetter R. J., Lamar C. H. Abnormal response of tumor vasculature to vasoactive drugs. J Natl Cancer Inst. 1984 Jan;72(1):145–150. doi: 10.1093/jnci/72.1.145. [DOI] [PubMed] [Google Scholar]
- Clifton K. H., Jirtle R. Mammary carcinoma cell population growth in preirradiated and unirradiated transplant sites. Viable tumor growth, vascularity, and the tumor-bed effect. Radiology. 1975 Nov;117(2):459–465. doi: 10.1148/117.2.459. [DOI] [PubMed] [Google Scholar]
- Cornbleet M. A., McElwain T. J., Kumar P. J., Filshie J., Selby P., Carter R. L., Hedley D. W., Clark M. L., Millar J. L. Treatment of advanced malignant melanoma with high-dose melphalan and autologous bone marrow transplantation. Br J Cancer. 1983 Sep;48(3):329–334. doi: 10.1038/bjc.1983.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debreczeni L. A., Farsang C., Takács L. Effect of phenoxybenzamine, propranolol, phenylephrine and isoproterenol on the circulation of rats bearing Guérin carcinoma. Acta Physiol Acad Sci Hung. 1980;56(4):341–365. [PubMed] [Google Scholar]
- Foster D. O., Frydman M. L. Comparison of microspheres and 86Rb+ as tracers of the distribution of cardiac output in rats indicates invalidity of 86Rb+-based measurements. Can J Physiol Pharmacol. 1978 Feb;56(1):97–109. doi: 10.1139/y78-014. [DOI] [PubMed] [Google Scholar]
- Furner R. L., Brown R. K. L-phenylalanine mustard (L-PAM): the first 25 years. Cancer Treat Rep. 1980 Apr-May;64(4-5):559–574. [PubMed] [Google Scholar]
- Hedley D. W., McElwain T. J., Millar J. L., Gordon M. Y. Acceleration of bone-marrow recovery by pre-treatment with cyclophosphamide in patients receiving high-dose melphalan. Lancet. 1978 Nov 4;2(8097):966–968. doi: 10.1016/s0140-6736(78)92529-1. [DOI] [PubMed] [Google Scholar]
- Honn K. V., Onoda J. M., Pampalona K., Battaglia M., Neagos G., Taylor J. D., Diglio C. A., Sloane B. F. Inhibition by dihydropyridine class calcium channel blockers of tumor cell-platelet-endothelial cell interactions in vitro and metastasis in vivo. Biochem Pharmacol. 1985 Jan 15;34(2):235–241. doi: 10.1016/0006-2952(85)90130-3. [DOI] [PubMed] [Google Scholar]
- Jirtle R., Clifton K. H., Rankin J. H. Effects of several vasoactive drugs on the vascular resistance of MT-W9B tumors in W/Fu rats. Cancer Res. 1978 Aug;38(8):2385–2390. [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Shrivastav S., Jirtle R. L. Blood flow to primary tumors and lymph node metastases in SMT-2A tumor-bearing rats following intravenous flunarizine. Cancer Res. 1984 Mar;44(3):896–899. [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Shrivastav S., Shand D. G., Jirtle R. L. Effect of verapamil on malignant tissue blood flow in SMT-2A tumor-bearing rats. Cancer Res. 1982 Oct;42(10):3944–3949. [PubMed] [Google Scholar]
- Mattsson J., Lilja J., Peterson H. I. Influence of vasoactive drugs on local tumor blood flow. Eur J Cancer Clin Oncol. 1982 Jul;18(7):677–684. doi: 10.1016/0277-5379(82)90214-0. [DOI] [PubMed] [Google Scholar]
- Mendell P. L., Hollenberg N. K. Cardiac output distribution in the rat: comparison of rubidium and microsphere methods. Am J Physiol. 1971 Dec;221(6):1617–1620. doi: 10.1152/ajplegacy.1971.221.6.1617. [DOI] [PubMed] [Google Scholar]
- Meredith P. A., Elliott H. L., Pasanisi F., Kelman A. W., Sumner D. J., Reid J. L. Verapamil pharmacokinetics and apparent hepatic and renal blood flow. Br J Clin Pharmacol. 1985 Aug;20(2):101–106. doi: 10.1111/j.1365-2125.1985.tb05038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. L., Blackett N. M., Hudspith B. N. Enhanced post-irradiation recovery of the haemopoietic system in animals pretreated with a variety of cytotoxic agents. Cell Tissue Kinet. 1978 Sep;11(5):543–553. doi: 10.1111/j.1365-2184.1978.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Millar J. L., Hudspith B. N., McElwain T. J., Phelps T. A. Effect of high-dose melphalan on marrow and intestinal epithelium in mice pretreated with cyclophosphamide. Br J Cancer. 1978 Jul;38(1):137–142. doi: 10.1038/bjc.1978.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. L., Phelps T. A., Carter R. L., McElwain T. J. Cyclophosphamide pretreatment reduces the toxic effect of high dose melphalan on intestinal epithelium in sheep. Eur J Cancer. 1978 Nov;14(11):1283–1285. doi: 10.1016/0014-2964(78)90236-0. [DOI] [PubMed] [Google Scholar]
- Nugent L. J., Jain R. K. Extravascular diffusion in normal and neoplastic tissues. Cancer Res. 1984 Jan;44(1):238–244. [PubMed] [Google Scholar]
- Robinson B. A., Clutterbuck R. D., Millar J. L., McElwain T. J. Verapamil potentiation of melphalan cytotoxicity and cellular uptake in murine fibrosarcoma and bone marrow. Br J Cancer. 1985 Dec;52(6):813–822. doi: 10.1038/bjc.1985.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAPIRSTEIN L. A. Regional blood flow by fractional distribution of indicators. Am J Physiol. 1958 Apr;193(1):161–168. doi: 10.1152/ajplegacy.1958.193.1.161. [DOI] [PubMed] [Google Scholar]
- Selby P. J., Thomas J. M., Monaghan P., Sloane J., Peckham M. J. Human tumour xenografts established and serially transplanted in mice immunologically deprived by thymectomy, cytosine arabinoside and whole-body irradiation. Br J Cancer. 1980 Jan;41(1):52–61. doi: 10.1038/bjc.1980.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel G. G., Courtenay V. D., Rostom A. Y. Improved immune-suppression techniques for the exongrafting of human tumours. Br J Cancer. 1978 Feb;37(2):224–230. doi: 10.1038/bjc.1978.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. H., Antman E. M., Muller J. E., Braunwald E. Calcium channel blocking agents in the treatment of cardiovascular disorders. Part II: Hemodynamic effects and clinical applications. Ann Intern Med. 1980 Dec;93(6):886–904. doi: 10.7326/0003-4819-93-6-886. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Hori K., Abe I., Saito S., Sato H. Functional characterization of the microcirculation in tumors. Cancer Metastasis Rev. 1984;3(2):115–126. doi: 10.1007/BF00047659. [DOI] [PubMed] [Google Scholar]
- Van Nueten J. M., Vanhoutte P. M. Calcium entry blockers and vascular smooth muscle heterogeneity. Fed Proc. 1981 Dec;40(14):2862–2865. [PubMed] [Google Scholar]
- Wetterlin S., Aronsen K. F., Björkman I., Ahlgren I. Studies on methods for determination of the distribution of cardiac output in the mouse. Scand J Clin Lab Invest. 1977 Sep;37(5):451–454. [PubMed] [Google Scholar]
- Zanelli G. D., Fowler J. F. The measurement of blood perfusion in experimental tumors by uptake of 86Rb. Cancer Res. 1974 Jun;34(6):1451–1456. [PubMed] [Google Scholar]
- Zanelli G. D., Lucas P. B., Fowler J. F. The effect of anaesthetics on blood perfusion in transplanted mouse tumours. Br J Cancer. 1975 Sep;32(3):380–390. doi: 10.1038/bjc.1975.238. [DOI] [PMC free article] [PubMed] [Google Scholar]


