Abstract
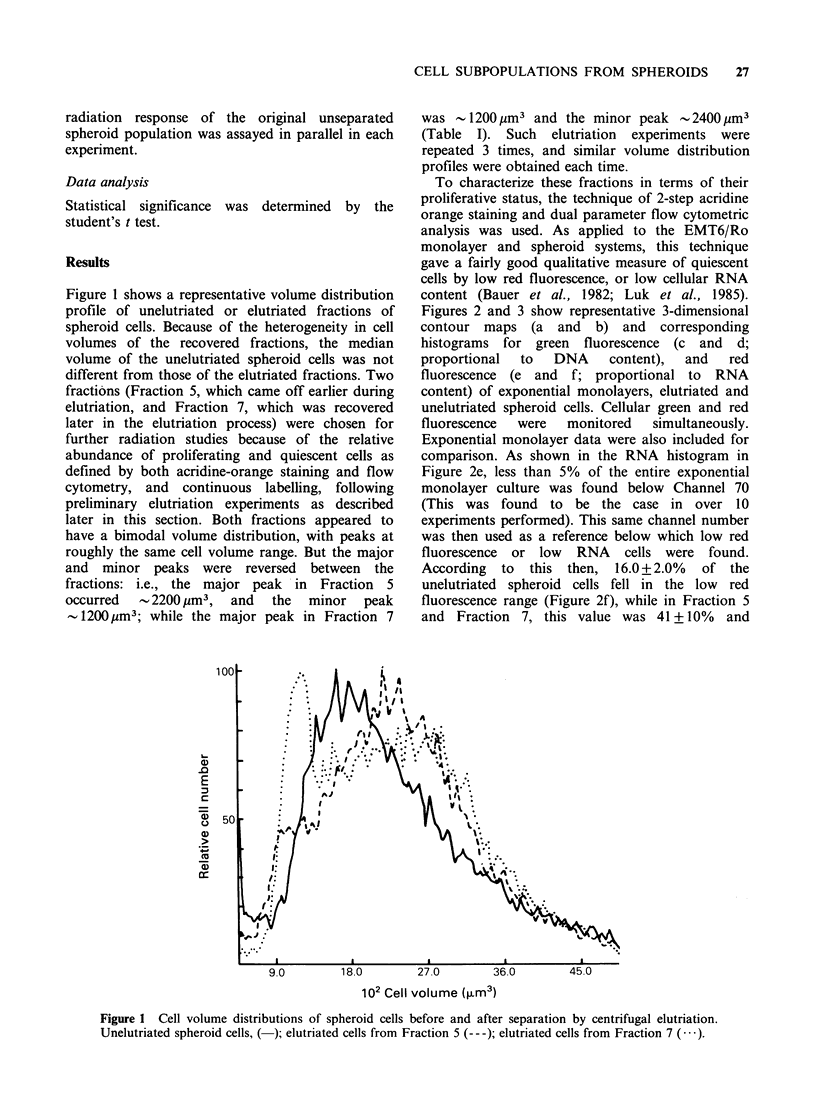
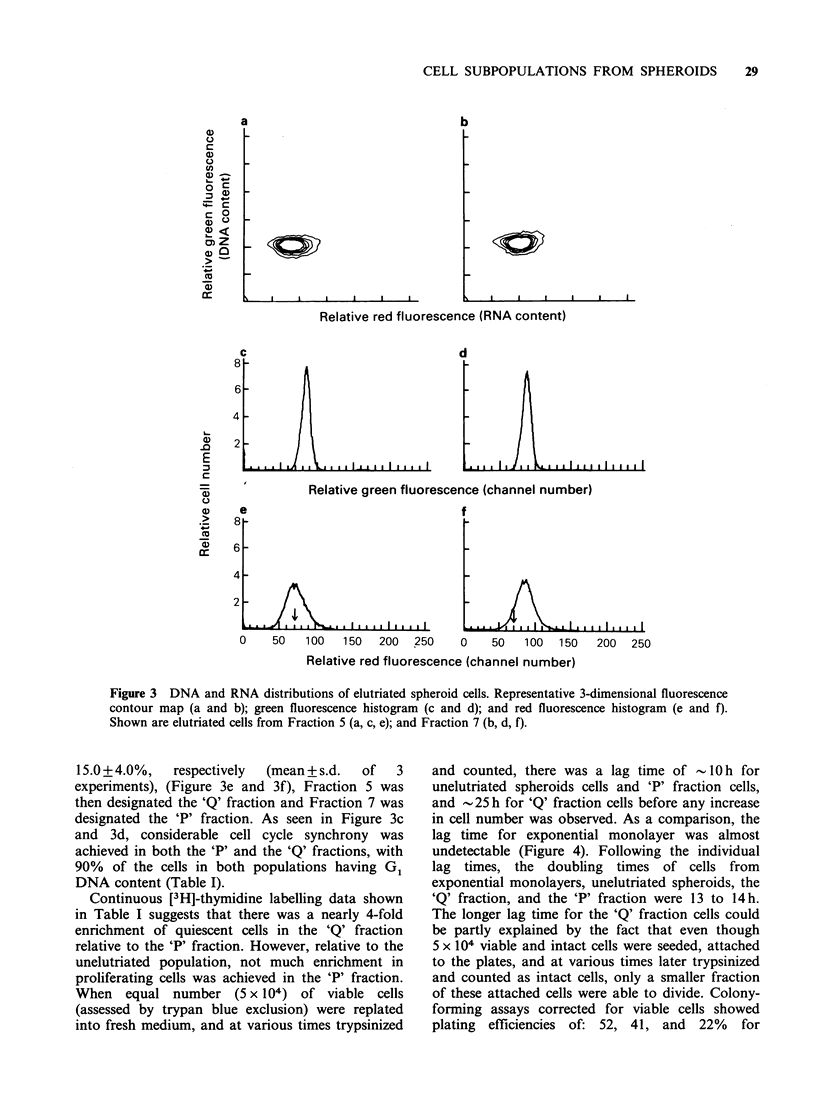
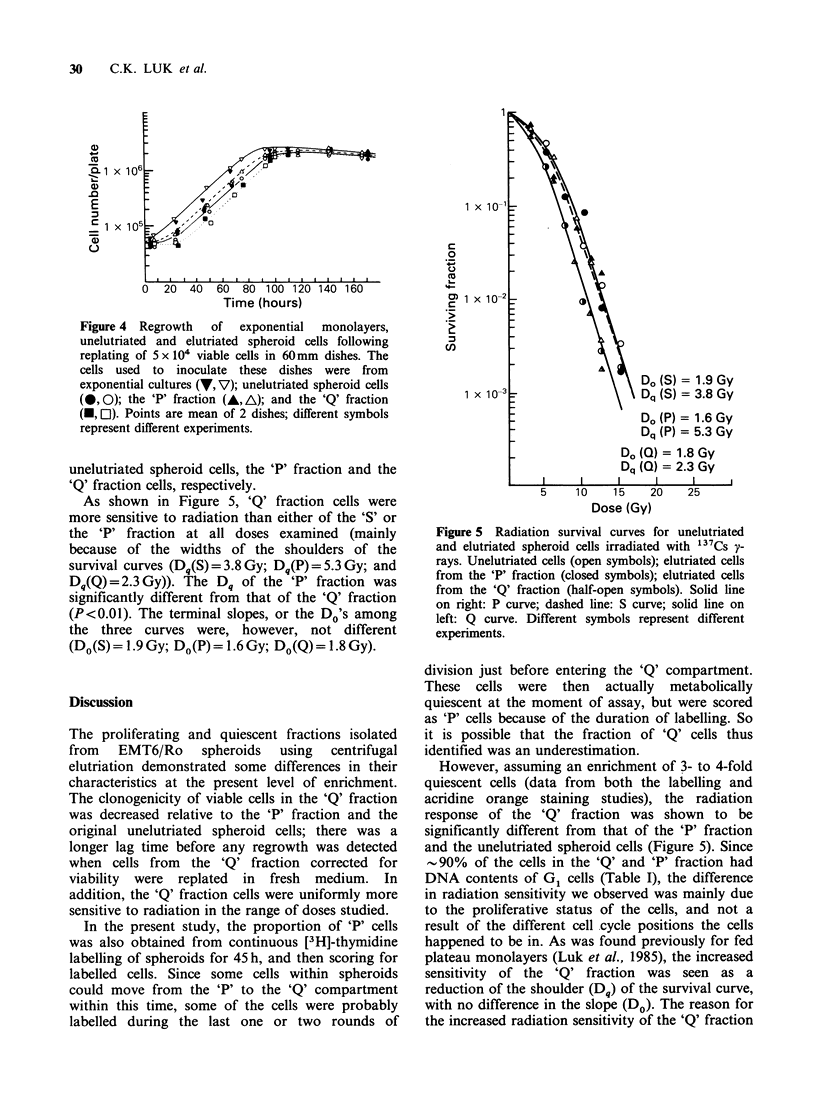
Two subpopulations enriched in cells with a G1-like content of DNA were isolated from EMT6/Ro spheroids using centrifugal elutriation. The techniques of two-step acridine orange staining followed by flow cytometry, and continuous [3H]-thymidine labelling agreed qualitatively that one of these subpopulations predominantly consisted of proliferating G1 cells, while the other contained about four times more quiescent G0/G1 cells. These two subpopulations had similar median cell volumes and DNA contents, but the cell volume distributions were different. The clonogenicity was greater in the 'proliferating' subpopulation than the 'quiescent' subpopulation. When cell number seeded was corrected for viability, regrowth studies showed that there was a longer time (25 h) for the 'quiescent' subpopulation than the 'proliferating' subpopulation (10 h) before any increase in cell number was observed. In addition, relative to the 'proliferating' cells, the 'quiescent' cells were more sensitive when exposed to 137Cs gamma-ray radiation. The D0's were similar between the two subpopulations (D0 = 1.6 Gy and 1.8 Gy for the 'proliferating' G1 and 'quiescent' G0/G1 subpopulation, respectively), but the width of the shoulder of the radiation survival curve was reduced in the 'quiescent' subpopulation (Dq = 2.3 Gy vs. 5.3 Gy).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer K. D., Keng P. C., Sutherland R. M. Isolation of quiescent cells from multicellular tumor spheroids using centrifugal elutriation. Cancer Res. 1982 Jan;42(1):72–78. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Melamed M. R. New cell cycle compartments identified by multiparameter flow cytometry. Cytometry. 1980 Sep;1(2):98–108. doi: 10.1002/cyto.990010203. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Bucana C. Mechanism of tumor cell resistance to lysis by syngeneic lymphocytes. Cancer Res. 1977 Nov;37(11):3945–3956. [PubMed] [Google Scholar]
- Hermens A. F., Barendsen G. W. The proliferative status and clonogenic capacity of tumour cells in a transplantable rhabdomyosarcoma of the rat before and after irradiation with 800 rad of X-rays. Cell Tissue Kinet. 1978 Jan;11(1):83–100. doi: 10.1111/j.1365-2184.1978.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Keng P. C., Li C. K., Wheeler K. T. Synchronization of 9L rat brain tumor cells by centrifugal elutriation. Cell Biophys. 1980 Sep;2(3):191–206. doi: 10.1007/BF02790449. [DOI] [PubMed] [Google Scholar]
- Luk C. K., Keng P. C., Sutherland R. M. Regrowth and radiation sensitivity of quiescent cells isolated from EMT6/Ro-fed plateau monolayers. Cancer Res. 1985 Mar;45(3):1020–1025. [PubMed] [Google Scholar]
- Potmesil M., Goldfeder A. Cell kinetics of irradiated experimental tumors: cell transition from the non-proliferating to the proliferating pool. Cell Tissue Kinet. 1980 Sep;13(5):563–570. doi: 10.1111/j.1365-2184.1980.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Durand R. E. Radiation response of multicell spheroids--an in vitro tumour model. Curr Top Radiat Res Q. 1976 Jan;11(1):87–139. [PubMed] [Google Scholar]
- Sutherland R. M., McCredie J. A., Inch W. R. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst. 1971 Jan;46(1):113–120. [PubMed] [Google Scholar]
- Sutherland R. M. Selective chemotherapy of noncycling cells in an in vitro tumor model. Cancer Res. 1974 Dec;34(12):3501–3503. [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Sharpless T., Melamed M. R. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977 Jan;25(1):46–56. doi: 10.1177/25.1.64567. [DOI] [PubMed] [Google Scholar]
- Valeriote F., van Putten L. Proliferation-dependent cytotoxicity of anticancer agents: a review. Cancer Res. 1975 Oct;35(10):2619–2630. [PubMed] [Google Scholar]
- Wallen C. A., Ridinger D. N., Dethlefsen L. A. Heterogeneity of X-ray cytotoxicity in proliferating and quiescent murine mammary carcinoma cells. Cancer Res. 1985 Jul;45(7):3064–3069. [PubMed] [Google Scholar]
- Warters R. L., Lyons B. W., Ridinger D. N., Dethlefsen L. A. DNA damage repair in quiescent murine mammary carcinoma cells in culture. Biochim Biophys Acta. 1985 Apr 19;824(4):357–364. doi: 10.1016/0167-4781(85)90043-0. [DOI] [PubMed] [Google Scholar]


