Abstract
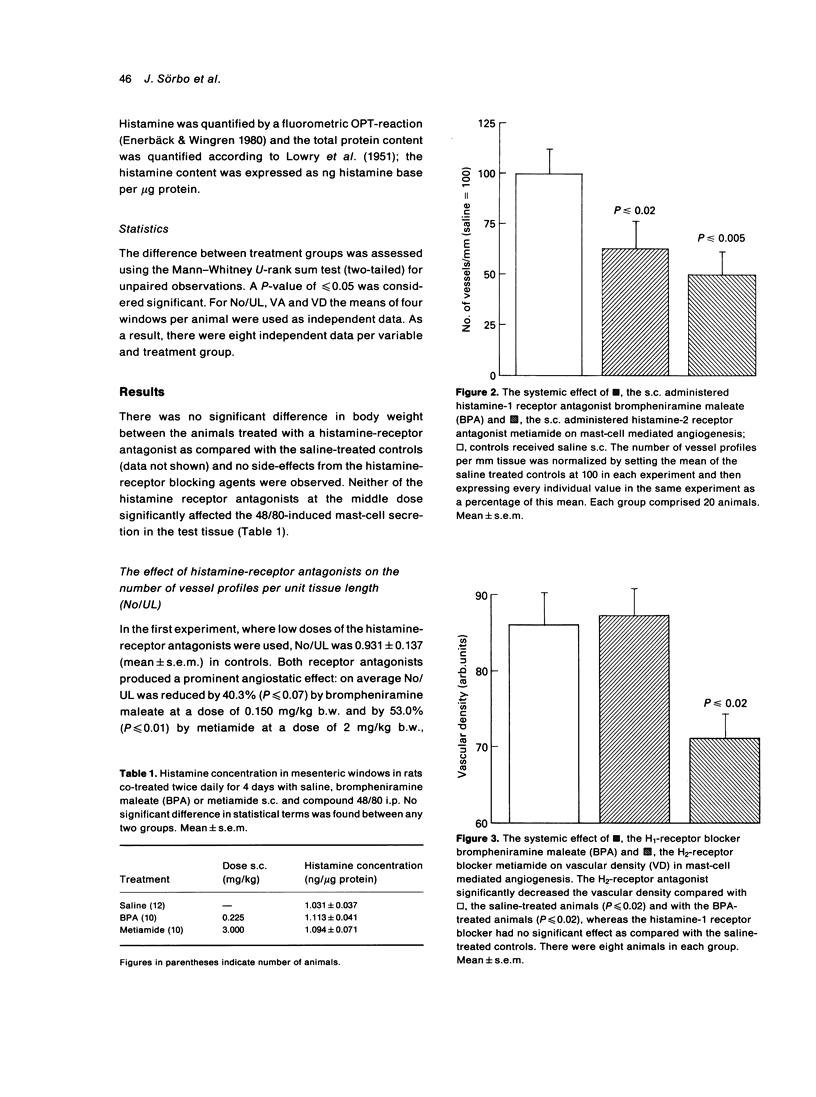
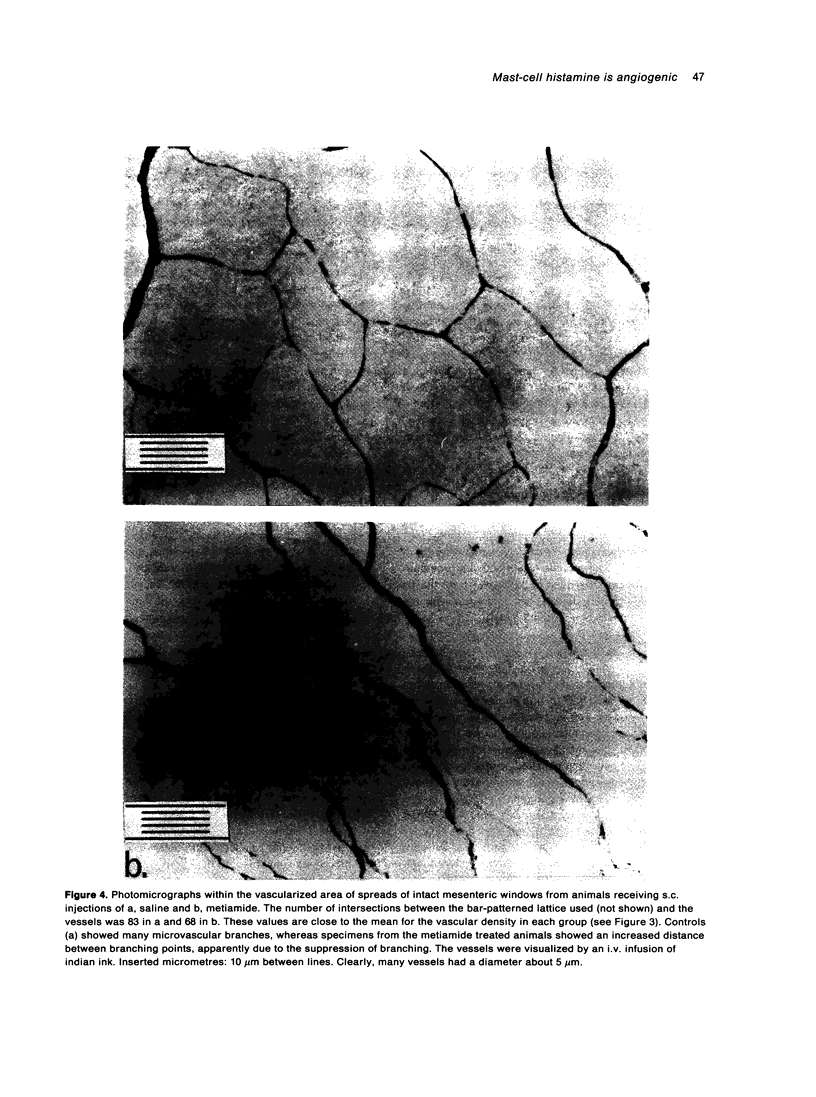
The activation of mast-cells in situ induces angiogenesis in normally vascularized, adult mammalian tissue. Since the secreting mast-cell characteristically releases histamine, we studied the possible role of histamine in the outcome of mast-cell mediated angiogenesis using the rat mesenteric window assay. One H1-receptor antagonist, brompheniramine maleate (BPA), and one H2-receptor antagonist, metiamide, were separately administered systemically (s.c.) at non-toxic doses during the period of angiogenesis induction. Angiogenesis was effected by i.p. injections of the mast-cell secretagogue compound 48/80 for 5 consecutive days. The animals were killed 14 days after the start of the i.p. and s.c. treatment, close to the middle of the expanding angiogenic phase of the angiogenic reaction studied. Angiogenesis was quantified in terms of (a) the number of vessel profiles per unit tissue length (No/UL), which reflects mainly the degree of branching and/or tortuosity, (b) the relative vascularized area (VA), which is a measure of spatial extension, and (c) the vascular density (VD), a measure of vessel density per unit area of vascularized tissue. Whereas BPA significantly suppressed No/UL, metiamide significantly reduced No/UL and VD in statistical terms suggesting that endogenous mast-cell histamine is angiogenic through both H1- and H2-receptors. This appears to be the first paper to report that the occupancy of H2-receptors is angiogenic.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black J. W., Duncan W. A., Emmett J. C., Ganellin C. R., Hesselbo T., Parsons M. E., Wyllie J. H. Metiamide--an orally active histamine H2-receptor antagonist. Agents Actions. 1973 Oct;3(3):133–137. doi: 10.1007/BF01965723. [DOI] [PubMed] [Google Scholar]
- Clinton M., Long W. F., Williamson F. B., Duncan J. I., Thompson W. D. Effect of the mast cell activator compound 48/80 and heparin on angiogenesis in the chick chorioallantoic membrane. Int J Microcirc Clin Exp. 1988 Nov;7(4):315–326. [PubMed] [Google Scholar]
- Cowen T., Trigg P., Eady R. A. Distribution of mast cells in human dermis: development of a mapping technique. Br J Dermatol. 1979 Jun;100(6):635–640. doi: 10.1111/j.1365-2133.1979.tb08066.x. [DOI] [PubMed] [Google Scholar]
- Dabbous M. K., Woolley D. E., Haney L., Carter L. M., Nicolson G. L. Host-mediated effectors of tumor invasion: role of mast cells in matrix degradation. Clin Exp Metastasis. 1986 Apr-Jun;4(2):141–152. doi: 10.1007/BF00119080. [DOI] [PubMed] [Google Scholar]
- Duncan J. I., Brown F. I., McKinnon A., Long W. F., Williamson F. B., Thompson W. D. Patterns of angiogenic response to mast cell granule constituents. Int J Microcirc Clin Exp. 1992 Feb;11(1):21–33. [PubMed] [Google Scholar]
- Enerbäck L., Franzén L., Norrby K. A tissue model for the study of cell proliferation parameters in vivo. Histochemistry. 1976 Jun 28;47(3):207–218. doi: 10.1007/BF00489963. [DOI] [PubMed] [Google Scholar]
- Enerbäck L., Wingren U. Histamine content of peritoneal and tissue mast cells of growing rats. Histochemistry. 1980;66(2):113–124. doi: 10.1007/BF00494639. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990 Jan 3;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Franzén, Norrby K. Local mitogenic effect of tissue mast cell secretion. Cell Tissue Kinet. 1980 Nov;13(6):635–642. doi: 10.1111/j.1365-2184.1980.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Fraser R. A., Simpson J. G. Role of mast cells in experimental tumour angiogenesis. Ciba Found Symp. 1983;100:120–131. doi: 10.1002/9780470720813.ch8. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Burd P. R., Galli S. J. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990 Dec;11(12):458–464. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- Hobson B., Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984 Apr;49(4):405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordana M., Befus A. D., Newhouse M. T., Bienenstock J., Gauldie J. Effect of histamine on proliferation of normal human adult lung fibroblasts. Thorax. 1988 Jul;43(7):552–558. doi: 10.1136/thx.43.7.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M. Mediators of angiogenesis: the biological significance of basic fibroblast growth factor (bFGF)-heparin and heparan sulfate interactions. Semin Cancer Biol. 1992 Apr;3(2):81–87. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marks R. M., Roche W. R., Czerniecki M., Penny R., Nelson D. S. Mast cell granules cause proliferation of human microvascular endothelial cells. Lab Invest. 1986 Sep;55(3):289–294. [PubMed] [Google Scholar]
- Meininger C. J., Zetter B. R. Mast cells and angiogenesis. Semin Cancer Biol. 1992 Apr;3(2):73–79. [PubMed] [Google Scholar]
- Mellblom L., Enerbäck L. Protein content, dry mass and chemical composition of individual mast cells related to body growth. Histochemistry. 1979 Sep;63(2):129–143. doi: 10.1007/BF00644535. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Oda M., Kaneko K., Honda K., Komatsu H., Tsuchiya M. Radioautographic characterization of H1 and H2 receptor antagonists. Binding sites in rat gastric mucosal microcirculatory system. Adv Exp Med Biol. 1988;242:151–160. [PubMed] [Google Scholar]
- Nehls V., Denzer K., Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992 Dec;270(3):469–474. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- Norrby K. Effect of heparin, histamine and serotonin on the density-dependent inhibition of replication in two fibroblastic cell lines. Virchows Arch B Cell Pathol. 1973 Dec 31;15(1):75–93. doi: 10.1007/BF02889327. [DOI] [PubMed] [Google Scholar]
- Norrby K., Enerbäck L., Franzén L. Mast cell activation and tissue cell proliferation. Cell Tissue Res. 1976 Jul 30;170(3):289–303. doi: 10.1007/BF00219412. [DOI] [PubMed] [Google Scholar]
- Norrby K., Eneström S. Cellular and extracellular changes following mast-cell secretion in avascular rat mesentery. An electron-microscopic study. Cell Tissue Res. 1984;235(2):339–345. doi: 10.1007/BF00217858. [DOI] [PubMed] [Google Scholar]
- Norrby K. Evidence of mast-cell histamine being mitogenic in intact tissue. Agents Actions. 1985 Apr;16(3-4):287–290. doi: 10.1007/BF01983162. [DOI] [PubMed] [Google Scholar]
- Norrby K. Heparin and angiogenesis: a low-molecular-weight fraction inhibits and a high-molecular-weight fraction stimulates angiogenesis systemically. Haemostasis. 1993 Mar;23 (Suppl 1):141–149. doi: 10.1159/000216923. [DOI] [PubMed] [Google Scholar]
- Norrby K. Intradermal mast-cell secretion causing cutaneous mitogenesis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;42(3):263–269. doi: 10.1007/BF02890389. [DOI] [PubMed] [Google Scholar]
- Norrby K., Jakobsson A., Sörbo J. Mast-cell secretion and angiogenesis, a quantitative study in rats and mice. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(4):251–256. doi: 10.1007/BF02899089. [DOI] [PubMed] [Google Scholar]
- Norrby K., Jakobsson A., Sörbo J. Mast-cell-mediated angiogenesis: a novel experimental model using the rat mesentery. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(3):195–206. doi: 10.1007/BF02889963. [DOI] [PubMed] [Google Scholar]
- Norrby K., Jakobsson A., Sörbo J. On mast-cell-mediated angiogenesis in the rat mesenteric-window assay. Agents Actions. 1990 Apr;30(1-2):231–233. doi: 10.1007/BF01969046. [DOI] [PubMed] [Google Scholar]
- Norrby K., Jakobsson A., Sörbo J. Quantitative angiogenesis in spreads of intact rat mesenteric windows. Microvasc Res. 1990 May;39(3):341–348. doi: 10.1016/0026-2862(90)90047-u. [DOI] [PubMed] [Google Scholar]
- Norrby K. Mast cell histamine, a local mitogen acting via H2-receptors in nearby tissue cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;34(1):13–20. doi: 10.1007/BF02892403. [DOI] [PubMed] [Google Scholar]
- Norrby K., Sörbo J. Heparin enhances angiogenesis by a systemic mode of action. Int J Exp Pathol. 1992 Apr;73(2):147–155. [PMC free article] [PubMed] [Google Scholar]
- Rakusan K., Sarkar K., Turek Z., Wicker P. Mast cells in the rat heart during normal growth and in cardiac hypertrophy. Circ Res. 1990 Feb;66(2):511–516. doi: 10.1161/01.res.66.2.511. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A., Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J Submicrosc Cytol Pathol. 1989 Jan;21(1):1–34. [PubMed] [Google Scholar]
- Shing Y., Folkman J., Haudenschild C., Lund D., Crum R., Klagsbrun M. Angiogenesis is stimulated by a tumor-derived endothelial cell growth factor. J Cell Biochem. 1985;29(4):275–287. doi: 10.1002/jcb.240290402. [DOI] [PubMed] [Google Scholar]
- Smaje L. H., Noor N. M., Clough G. F. Changing sensitivity to H1 and H2 receptor antagonists in the growing vasculature. Adv Exp Med Biol. 1988;242:145–150. doi: 10.1007/978-1-4684-8935-4_17. [DOI] [PubMed] [Google Scholar]
- Thompson W. D., Brown F. I. Quantitation of histamine-induced angiogenesis in the chick chorioallantoic membrane: mode of action of histamine is indirect. Int J Microcirc Clin Exp. 1987 Dec;6(4):343–357. [PubMed] [Google Scholar]
- Zauberman H., Michaelson I. C., Bergmann F., Maurice D. M. Stimulation of neovascularization of the cornea by biogenic amines. Exp Eye Res. 1969 Jan;8(1):77–83. doi: 10.1016/s0014-4835(69)80083-7. [DOI] [PubMed] [Google Scholar]