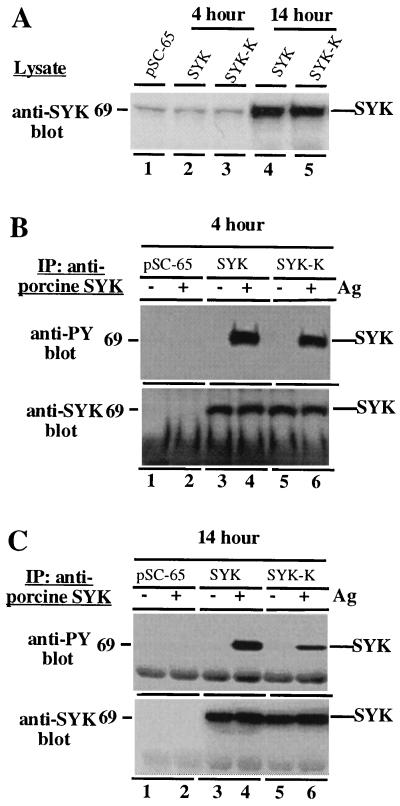
Figure 2.
syk tyrosine phosphorylation is generated primarily by syk transphosphorylation and phosphorylation by other kinases. (A) Relative expression of syk with 4-h or 14-h vaccinia infections. RBL-2H3 cells were infected with the indicated viruses for either 4 h (lanes 1–3) or 14 h (lanes 4 and 5). Cells were then lysed and analyzed by immunoblotting with an anti-syk antibody capable of recognizing both rat and porcine syk. This experiment was done as a pilot experiment to determine the expression time course of syk after infection. The experiments below were done separately using the conditions defined in this experiment. (B) Tracer expression of wild-type syk or syk-K results in similar levels of Fc receptor-induced tyrosine phosphorylation. RBL-2H3 cells were loaded with IgE, infected for 4 h with the indicated recombinant vaccinia viruses, stimulated or not with antigen, and lysed. (Upper) Lysates were subjected to precipitation with anti-porcine syk antibody and analyzed by anti-phosphotyrosine immunoblotting. (Lower) The blot was then stripped and reprobed by anti-syk immunoblotting to verify equivalent amounts of syk protein in each lane. (C) High level overexpression of wild-type syk or syk-K results in substantially reduced tyrosine phosphorylation of syk-K relative to wild-type syk. Identical experiment to above except that RBL-2H3 cells were infected for 14 h with the indicated recombinant vaccinia viruses. (Upper) Lysates were subjected to precipitation with anti-porcine syk antibody and analyzed by anti-phosphotyrosine immunoblotting. (Lower) The blot was then stripped and reprobed by anti-syk immunoblotting to verify equivalent amounts of syk protein in each lane.