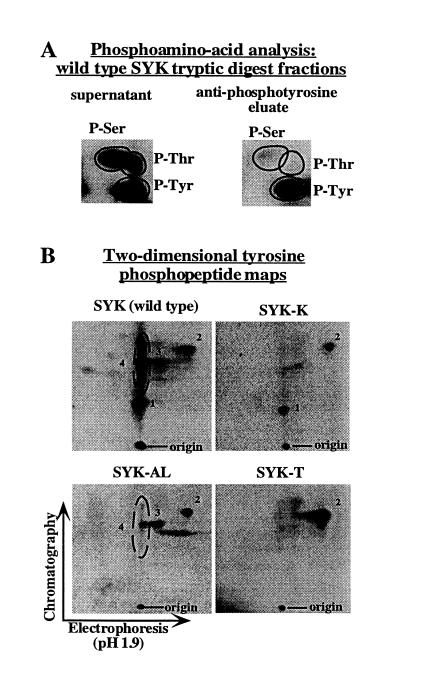
Figure 4.
Phosphopeptide analysis of syk tyrosine phosphorylation sites. Tryptic peptides derived from 32P-labeled wild-type syk, syk-K, syk-AL, and syk-T were generated as described, and then immunoprecipitated with anti-phosphotyrosine to generate a population of bound peptides (eluate fraction) and unbound peptides (supernatant fraction). (A) Anti-phosphotyrosine immunoprecipitation of tryptic peptides effectively eliminates serine/threonine phosphorylated peptides from the bound peptides (eluate fraction). Phosphoamino acid analysis was performed on aliquots of the supernatant (Left) or eluate (Right) fractions from the anti-phosphotyrosine immunoprecipitation of wild-type syk tryptic peptides. (B) Two-dimensional phosphopeptide analyses of the eluate fractions. For all syk constructs, equal amounts of eluate fractions were subjected to two-dimensional phosphopeptide mapping. The set of spots inside the ellipse in the wild-type syk map are grouped together as “peptide 4” because over several experiments the labeling of the spots above and below the main spot was variable. We believe that these spots are isoforms of a single peptide generated by contaminating protease activity in the trypsin preparation as we have previously described (19). The increased intensity of peptide 2 phosphorylation in the syk-T map relative to the other maps is reproducible, but is of unclear origin.