Abstract
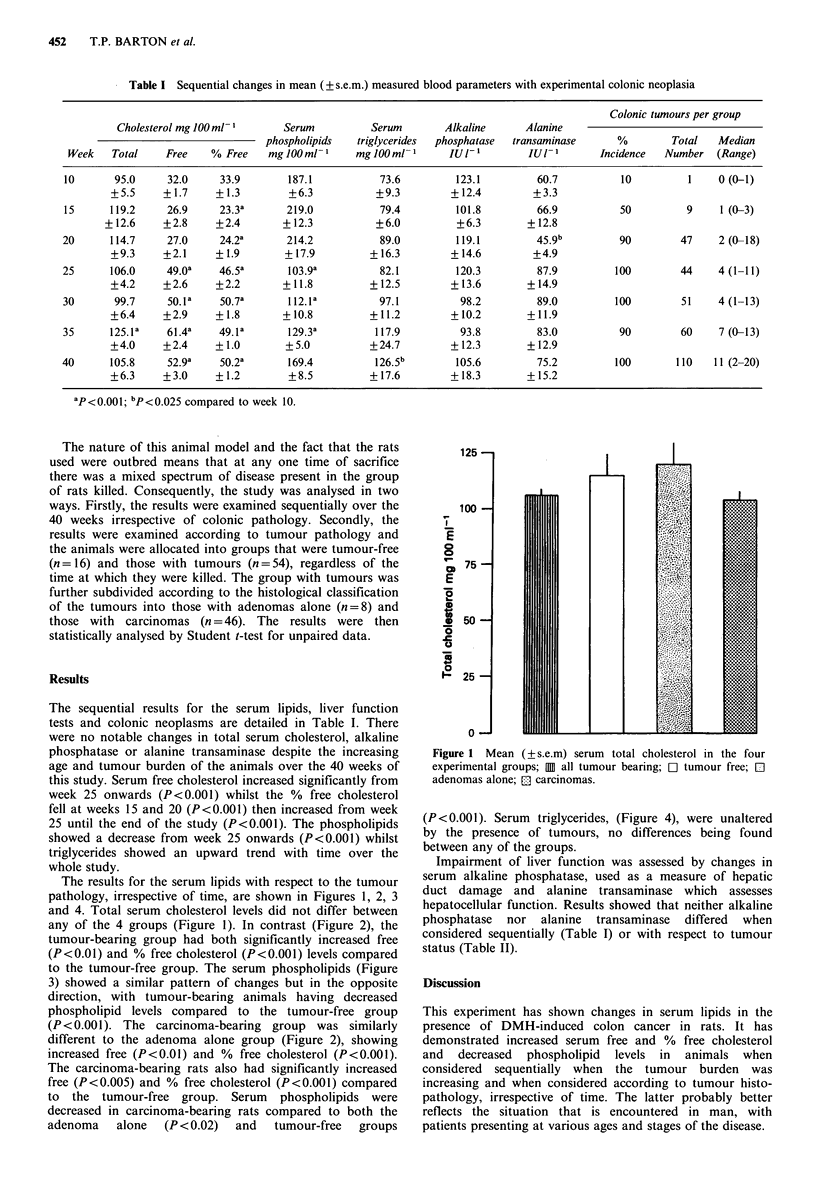
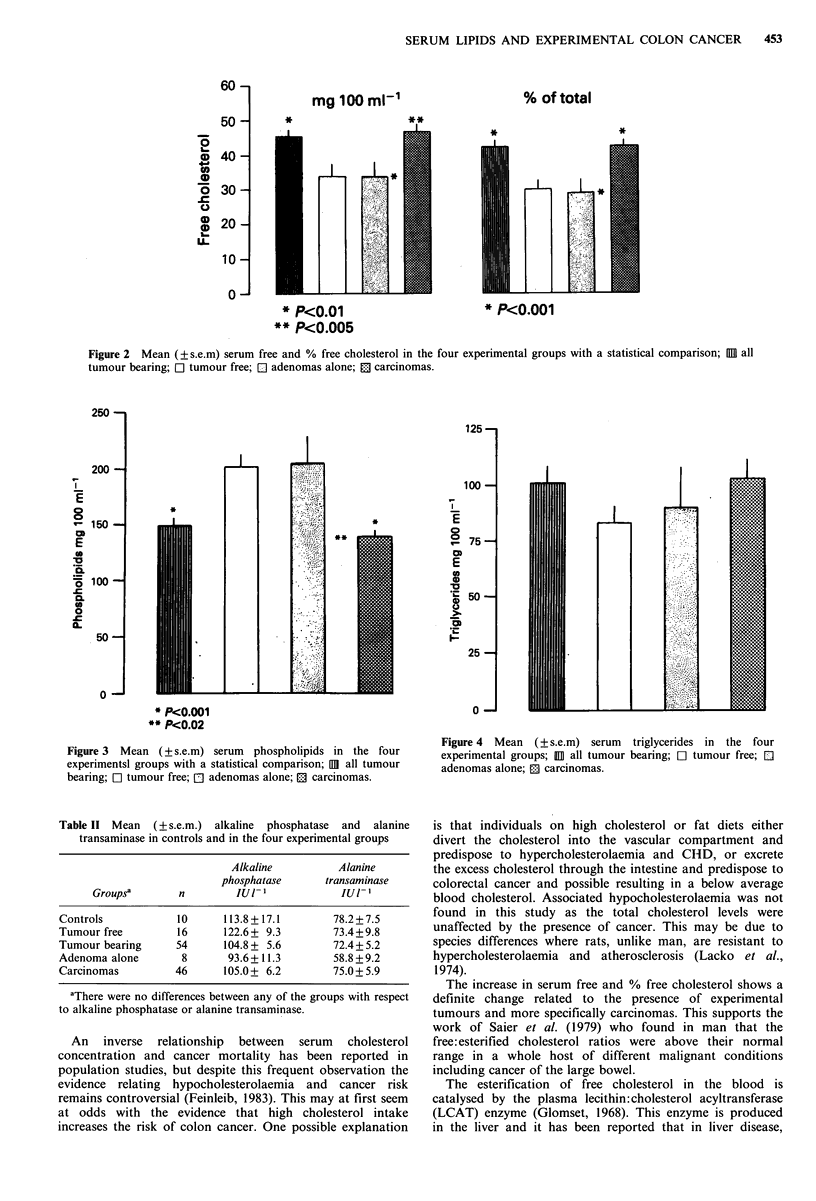
People at risk from coronary heart disease and large bowel cancer are drawn from the same urbanised, industrialised Western populations. Whilst changes in blood lipids are well recognised in heart disease, little is known of their role in large bowel cancer. This study investigates serial alterations in blood lipids in the 1,2-dimethylhydrazine (DMH) rat model of colon cancer. Eighty Wistar rats received a 5 weekly regimen of DMH. At week 10, and at 5 weekly intervals until week 40, random groups of 10 rats were killed and blood taken for total and free cholesterol, phospholipids, triglycerides and liver enzymes. All colonic neoplasms were histologically classified either as adenomas or carcinomas with groups being allocated into tumour-free (n = 16) or tumour-bearing (n = 54), the latter group being further sub-divided into animals with adenoma alone (n = 8) and those with carcinoma (n = 46). Results were considered both sequentially and according to tumour status. Sequential results showed that with increase in colonic neoplasms with time there were accompanying increases in free and % free cholesterol and in phospholipids (P less than 0.001). There were no changes in total cholesterol, triglycerides or liver enzymes. Results according to tumour status showed that whilst there was no difference in total cholesterol or triglycerides between tumour-free and tumour-bearing rats, there was a significant increase in free (P less than 0.01) and % free cholesterol (P less than 0.001) and a decrease in phospholipids in the tumour-bearing animals (P less than 0.001). There was no difference in any serum lipid between tumour-free and adenoma-bearing rats. In animals with carcinoma, while there was no difference in total cholesterol or triglycerides, there was an increase in free (P less than 0.005) and % free cholesterol (P less than 0.001) and a decrease in phospholipids (P less than 0.001) compared to tumour-free rats. The data show for the first time a clear relationship between blood lipids and the presence or absence of large bowel cancer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broitman S. A. Cholesterol excretion and colon cancer. Cancer Res. 1981 Sep;41(9 Pt 2):3738–3740. [PubMed] [Google Scholar]
- Calandra S., Martin M. J., McIntyre N. Plasma lecithin: cholesterol acyltransferase activity in liver disease. Eur J Clin Invest. 1971 May;1(5):352–360. doi: 10.1111/j.1365-2362.1971.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Cruse J. P., Lewin M. R., Clark C. G. Dietary cholesterol deprivation improves survival and reduces incidence of metastatic colon cancer in dimethylhydrazine-pretreated rats. Gut. 1982 Jul;23(7):594–599. doi: 10.1136/gut.23.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse J. P., Lewin M. R., Ferulano G. P., Clark C. G. Co-carcinogenic effects of dietary cholesterol in experimental colon cancer. Nature. 1978 Dec 21;276(5690):822–825. doi: 10.1038/276822a0. [DOI] [PubMed] [Google Scholar]
- Cruse P., Lewin M., Clark C. G. Dietary cholesterol is co-carcinogenic for human colon cancer. Lancet. 1979 Apr 7;1(8119):752–755. doi: 10.1016/s0140-6736(79)91209-1. [DOI] [PubMed] [Google Scholar]
- Doll R. Geographical variation in cancer incidence: a clue to causation. World J Surg. 1978 Sep;2(5):595–602. doi: 10.1007/BF01556055. [DOI] [PubMed] [Google Scholar]
- Filipe M. I. Mucous secretion in rat colonic mucosa during carcinogenesis induced by dimethylhydrazine. A morphological and histochemical study. Br J Cancer. 1975 Jul;32(1):60–77. doi: 10.1038/bjc.1975.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Hill M. J. The etiology of colon cancer. CRC Crit Rev Toxicol. 1975 Oct;4(1):31–82. doi: 10.1080/10408447509163834. [DOI] [PubMed] [Google Scholar]
- Lacko A. G., Rutenberg H. L., Soloff L. A. Serum cholesterol esterification in species resistant and susceptible to atherosclerosis. Atherosclerosis. 1974 Mar-Apr;19(2):297–305. doi: 10.1016/0021-9150(74)90064-1. [DOI] [PubMed] [Google Scholar]
- Lewis B. Plasma lipids and cancer. Biochem Soc Trans. 1983 Jun;11(3):252–254. doi: 10.1042/bst0110252. [DOI] [PubMed] [Google Scholar]
- Mannes G. A., Maier A., Thieme C., Wiebecke B., Paumgartner G. Relation between the frequency of colorectal adenoma and the serum cholesterol level. N Engl J Med. 1986 Dec 25;315(26):1634–1638. doi: 10.1056/NEJM198612253152602. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Jensen O. M., Parkin D. M., Zaridze D. G. Dietary and endogenous cholesterol and human cancer. Epidemiol Rev. 1984;6:192–216. doi: 10.1093/oxfordjournals.epirev.a036271. [DOI] [PubMed] [Google Scholar]
- Nydegger U. E., Butler R. E. Serum lipoprotein levels in patients with cancer. Cancer Res. 1972 Aug;32(8):1756–1760. [PubMed] [Google Scholar]
- Saier E. L., Nordstrand E., Juves M. W., Hartsock R. J. The ratio of free to esterified cholesterol in serum. A new discriminant in correlating lipid metabolism with disease state. Am J Clin Pathol. 1979 Jan;71(1):83–87. doi: 10.1093/ajcp/71.1.83. [DOI] [PubMed] [Google Scholar]
- Stähler F., Gruber W., Stinshoff K., Röschlau P. Eine praxisgerechte enzymatische Cholesterin-Bestimmung. Med Lab (Stuttg) 1977 Feb;30(2):29–37. [PubMed] [Google Scholar]
- Törnberg S. A., Holm L. E., Carstensen J. M., Eklund G. A. Risks of cancer of the colon and rectum in relation to serum cholesterol and beta-lipoprotein. N Engl J Med. 1986 Dec 25;315(26):1629–1633. doi: 10.1056/NEJM198612253152601. [DOI] [PubMed] [Google Scholar]
- Wynder E. L., Reddy B. S. Dietary fat and fiber and colon cancer. Semin Oncol. 1983 Sep;10(3):264–272. [PubMed] [Google Scholar]
- Wynder E. L., Shigematsu T. Environmental factors of cancer of the colon and rectum. Cancer. 1967 Sep;20(9):1520–1561. doi: 10.1002/1097-0142(196709)20:9<1520::aid-cncr2820200920>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Wynder E. L. The epidemiology of large bowel cancer. Cancer Res. 1975 Nov;35(11 Pt 2):3388–3394. [PubMed] [Google Scholar]


