Abstract
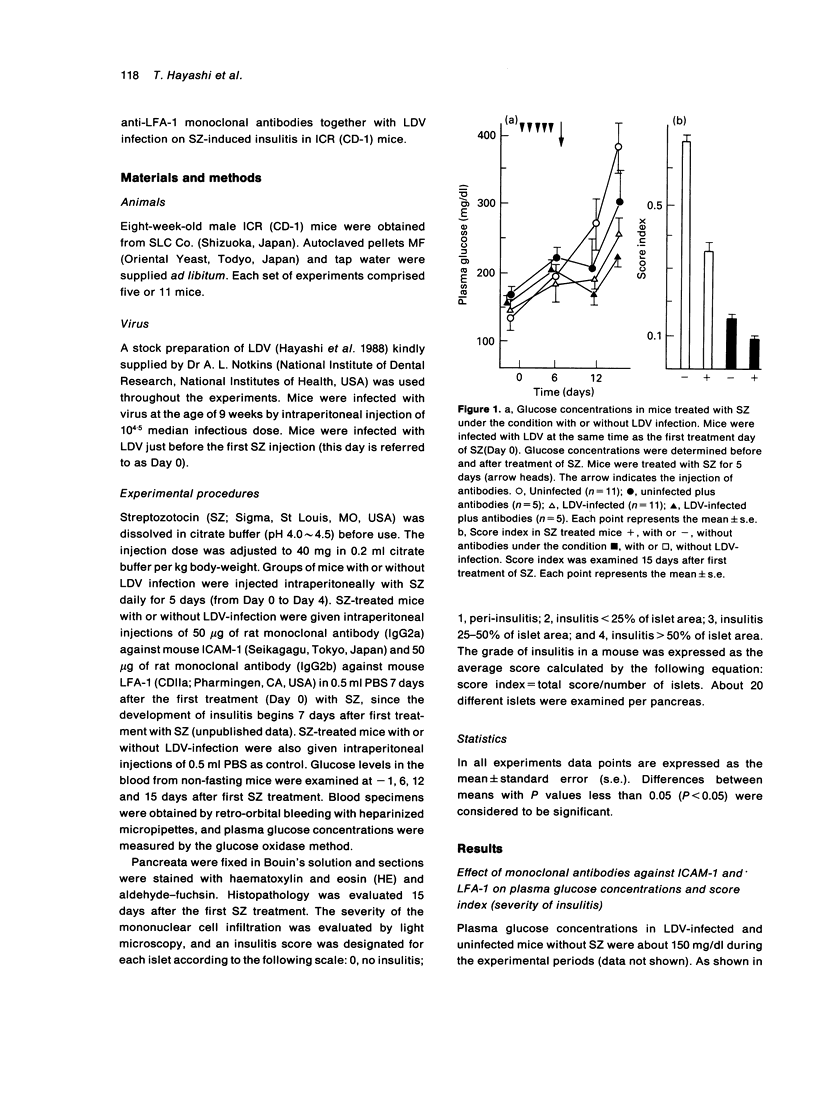
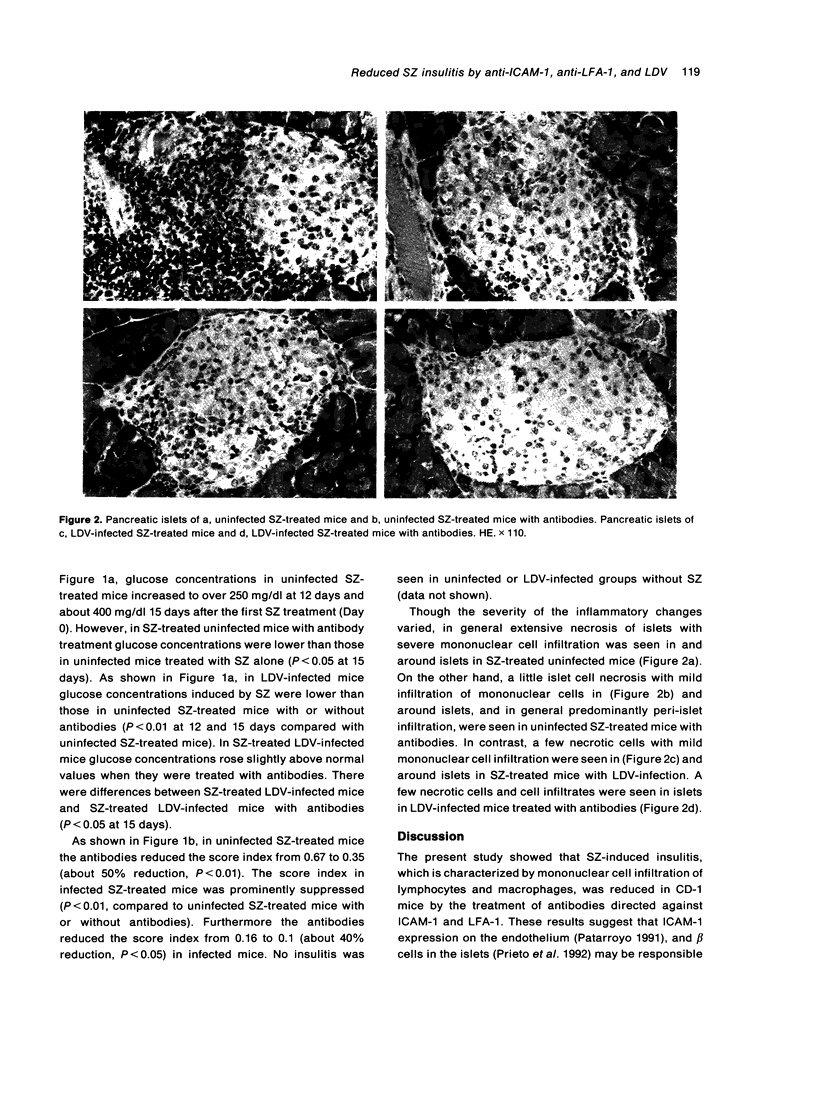
Multiple low-dose streptozotocin (SZ)-induced insulitis is an animal model for insulin-dependent diabetes mellitus characterized by a mononuclear cell infiltration. SZ-induced insulitis and blood glucose concentrations were reduced by treatment with anti-intercellular adhesion molecule-1(ICAM-1) and anti-lymphocyte function associated antigen-1 (LFA-1) monoclonal antibodies. This suppressing effect was also seen in mice infected with lactic dehydrogenase virus (LDV). These results suggest that the expression of ICAM-1 in islets and LFA-1 on mononuclear cells may be important in the development of SZ-induced insulitis. The suppressive effect of LDV infection on the development of insulitis is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanenberg H., Kolb-Bachofen V., Kantwerk-Funke G., Kolb H. Macrophage infiltration precedes and is a prerequisite for lymphocytic insulitis in pancreatic islets of pre-diabetic BB rats. Diabetologia. 1989 Feb;32(2):126–134. doi: 10.1007/BF00505185. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Iwata H., Hasegawa T., Ozaki M., Yamamoto H., Onodera T. Decrease in neutrophil migration induced by endotoxin and suppression of interleukin-1 production by macrophages in lactic dehydrogenase virus-infected mice. J Comp Pathol. 1991 Feb;104(2):161–170. doi: 10.1016/s0021-9975(08)80099-0. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Koike Y., Hasegawa T., Tsurudome M., Ozaki M., Yamamoto H., Onodera T. Inhibition of contact sensitivity by interferon in mice infected with lactic dehydrogenase virus. J Comp Pathol. 1991 May;104(4):357–366. doi: 10.1016/s0021-9975(08)80146-6. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Mori I., Yamamoto H. Lactic dehydrogenase virus infection prevents development of anti-nuclear antibody in (NZB x NZW)F1 mice; role of prostaglandin E2 and macrophage Ia antigen expression. Int J Exp Pathol. 1992 Oct;73(5):593–601. [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Noguchi Y., Kameyama Y. Suppression of development of anti-nuclear antibody and glomerulonephritis in NZB x NZWF1 mice by persistent infection with lactic dehydrogenase virus: possible involvement of superoxide anion as a progressive effector. Int J Exp Pathol. 1993 Dec;74(6):553–560. [PMC free article] [PubMed] [Google Scholar]
- Herold K. C., Montag A. G., Fitch F. W. Treatment with anti-T-lymphocyte antibodies prevents induction of insulitis in mice given multiple doses of streptozocin. Diabetes. 1987 Jul;36(7):796–801. doi: 10.2337/diab.36.7.796. [DOI] [PubMed] [Google Scholar]
- Hutchings P., Rosen H., O'Reilly L., Simpson E., Gordon S., Cooke A. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1990 Dec 13;348(6302):639–642. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Infection of mice with lactic dehydrogenase virus prevents development of experimental allergic encephalomyelitis. J Neuroimmunol. 1986 Mar;11(1):53–56. doi: 10.1016/0165-5728(86)90074-3. [DOI] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Live lactate dehydrogenase-elevating virus (LDV) induces suppressor T cells that inhibit the development of delayed hypersensitivity to LDV. J Gen Virol. 1986 Oct;67(Pt 10):2103–2112. doi: 10.1099/0022-1317-67-10-2103. [DOI] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Mouse Ia antigens are receptors for lactate dehydrogenase virus. Nature. 1984 May 3;309(5963):59–61. doi: 10.1038/309059a0. [DOI] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Pattern of infection and selective loss of Ia positive cells in suckling and adult mice inoculated with lactic dehydrogenase virus. Arch Virol. 1985;86(3-4):151–165. doi: 10.1007/BF01309821. [DOI] [PubMed] [Google Scholar]
- Isakov N., Feldman M., Segal S. Acute infection of mice with lactic dehydrogenase virus (LDV) impairs the antigen-presenting capacity of their macrophages. Cell Immunol. 1982 Jan 15;66(2):317–332. doi: 10.1016/0008-8749(82)90182-4. [DOI] [PubMed] [Google Scholar]
- Kolb-Bachofen V., Epstein S., Kiesel U., Kolb H. Low-dose streptozocin-induced diabetes in mice. Electron microscopy reveals single-cell insulitis before diabetes onset. Diabetes. 1988 Jan;37(1):21–27. doi: 10.2337/diab.37.1.21. [DOI] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976 Jul 30;193(4251):415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Muir A., Rovin B. H., Lacy P. E., Schreiner G. F. Macrophage-specific chemotactic lipid release by in vivo streptozocin-administered mouse islets. Diabetes. 1991 Nov;40(11):1459–1466. doi: 10.2337/diab.40.11.1459. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Nagafuchi S., Yamaguchi K., Takaki R. The role of thymic immunity and insulitis in the development of streptozocin-induced diabetes in mice. Diabetes. 1984 Sep;33(9):894–900. doi: 10.2337/diab.33.9.894. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Inhibition of antibodies to nuclear antigen and to DNA in New Zealand mice infected with lactate dehydrogenase virus. Science. 1972 Feb 18;175(4023):784–786. doi: 10.1126/science.175.4023.784. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Oschilewski U., Kiesel U., Kolb H. Administration of silica prevents diabetes in BB-rats. Diabetes. 1985 Feb;34(2):197–199. doi: 10.2337/diab.34.2.197. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. Leukocyte adhesion in host defense and tissue injury. Clin Immunol Immunopathol. 1991 Sep;60(3):333–348. doi: 10.1016/0090-1229(91)90091-n. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Prieto J., Kaaya E. E., Juntti-Berggren L., Berggren P. O., Sandler S., Biberfeld P., Patarroyo M. Induction of intercellular adhesion molecule-1 (CD54) on isolated mouse pancreatic beta cells by inflammatory cytokines. Clin Immunol Immunopathol. 1992 Dec;65(3):247–253. doi: 10.1016/0090-1229(92)90154-g. [DOI] [PubMed] [Google Scholar]
- Rowson K. E., Mahy B. W. Lactate dehydrogenase-elevating virus. J Gen Virol. 1985 Nov;66(Pt 11):2297–2312. doi: 10.1099/0022-1317-66-11-2297. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Rees J. C., Meltzer M. S. Macrophage function in tumor-bearing mice: evidence for lactic dehydrogenase-elevating virus-associated changes. J Immunol. 1980 Jun;124(6):2892–2899. [PubMed] [Google Scholar]
- Takei I., Asaba Y., Kasatani T., Maruyama T., Watanabe K., Yanagawa T., Saruta T., Ishii T. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J Autoimmun. 1992 Dec;5(6):665–673. doi: 10.1016/0896-8411(92)90184-r. [DOI] [PubMed] [Google Scholar]



