Abstract
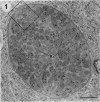
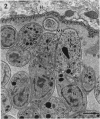
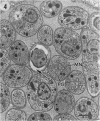
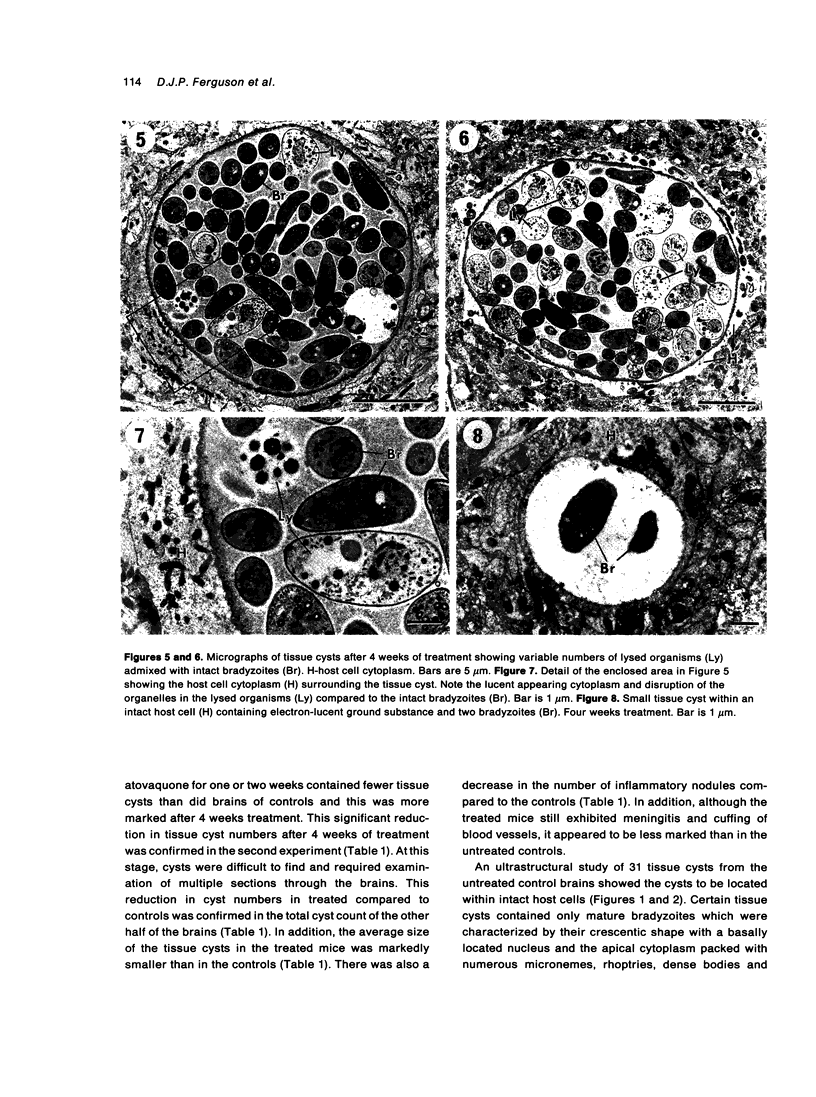
The morphological effects of drug treatment with atovaquone in the brains of mice chronically infected with Toxoplasma gondii was examined by light and electron microscopy. As early as 1 and 2 weeks of treatment there appeared to be fewer tissue cysts compared to untreated controls and this reduction was more significant after 4 weeks treatment. There also appeared to be a decrease in the number of inflammatory nodules and the severity of the meningitis. Ultrastructurally, the cysts of both treated and control animals were located within host cells. There was a marked increase in both the number of cysts with lysed bradyzoites and the number of degenerate bradyzoites after 4 weeks treatment. It is probable that the drug is more active against the metabolically active immature bradyzoites than the mature organisms. Drug treatment does not appear to result in rupture of tissue cysts or release of Toxoplasma antigens since there is a reduction rather than an increase in the inflammatory response. This drug may be useful in treating chronic toxoplasmosis since it appears to be active against the bradyzoites reducing the parasite burden (cyst number) without initiating a destructive inflammatory response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Huskinson-Mark J., Gutteridge W. E., Remington J. S. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother. 1992 Feb;36(2):326–330. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Huskinson J., Remington J. S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991 Feb;35(2):293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Lin T., Remington J. S. The activity of atovaquone (566C80) in murine toxoplasmosis is markedly augmented when used in combination with pyrimethamine or sulfadiazine. J Infect Dis. 1993 Feb;167(2):494–497. doi: 10.1093/infdis/167.2.494. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Hutchison W. M. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73(6):483–491. doi: 10.1007/BF00535321. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Hutchison W. M., Pettersen E. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. An immunocytochemical and ultrastructural study. Parasitol Res. 1989;75(8):599–603. doi: 10.1007/BF00930955. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Hutchison W. M. The host-parasite relationship of Toxoplasma gondii in the brains of chronically infected mice. Virchows Arch A Pathol Anat Histopathol. 1987;411(1):39–43. doi: 10.1007/BF00734512. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K., Escajadillo A. Cyst rupture as a pathogenic mechanism of toxoplasmic encephalitis. Am J Trop Med Hyg. 1987 May;36(3):517–522. doi: 10.4269/ajtmh.1987.36.517. [DOI] [PubMed] [Google Scholar]
- Hudson A. T., Dickins M., Ginger C. D., Gutteridge W. E., Holdich T., Hutchinson D. B., Pudney M., Randall A. W., Latter V. S. 566C80: a potent broad spectrum anti-infective agent with activity against malaria and opportunistic infections in AIDS patients. Drugs Exp Clin Res. 1991;17(9):427–435. [PubMed] [Google Scholar]
- Huskinson-Mark J., Araujo F. G., Remington J. S. Evaluation of the effect of drugs on the cyst form of Toxoplasma gondii. J Infect Dis. 1991 Jul;164(1):170–171. doi: 10.1093/infdis/164.1.170. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992 Aug;15(2):211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Petito C. K., Gold J. W., Cho E. S., Jordan B. D., Price R. W. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986 Mar;19(3):224–238. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Conley F. K., Remington J. S. Differences in virulence and development of encephalitis during chronic infection vary with the strain of Toxoplasma gondii. J Infect Dis. 1989 Apr;159(4):790–794. doi: 10.1093/infdis/159.4.790. [DOI] [PubMed] [Google Scholar]