Abstract
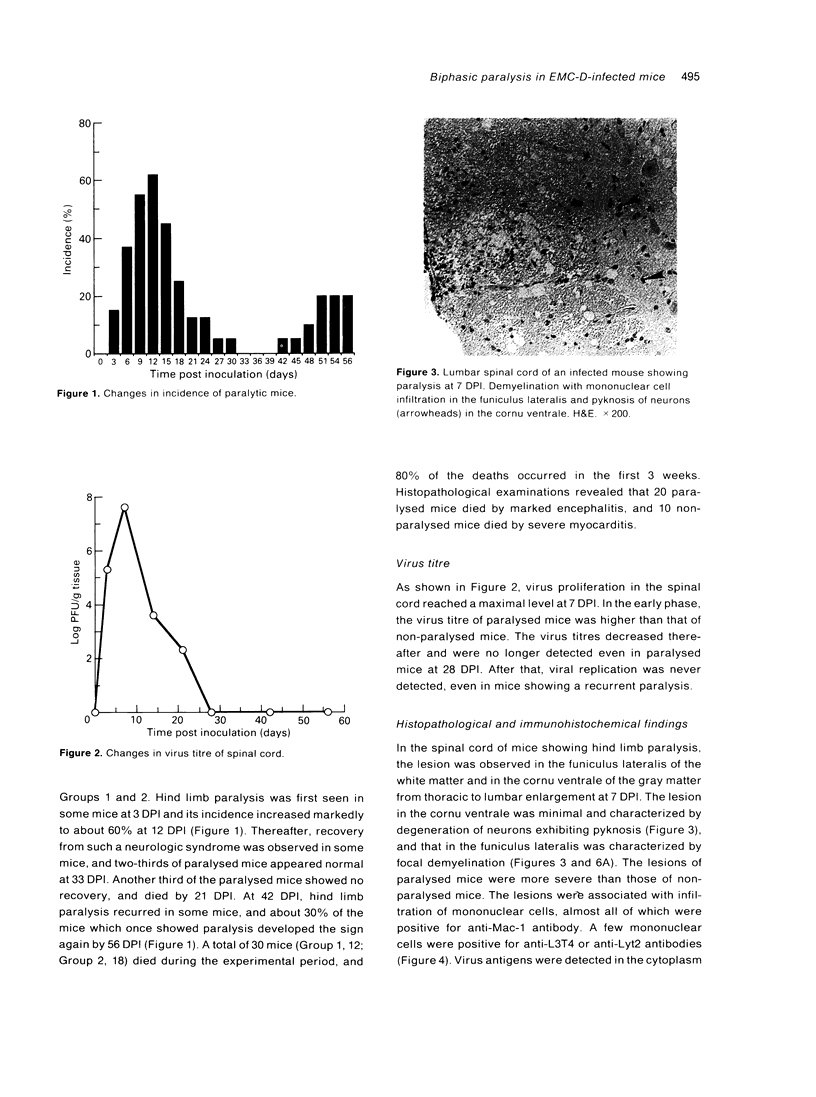
DBA/2 mice infected with the D variant of encephalomyocarditis virus (EMC-D) (10(1) PFU/head) developed biphasic hind limb paralysis. At 12 days post inoculation (12 DPI), 60% of the infected mice developed hind limb paralysis and two-thirds of them showed recovery by 33 DPI. Thereafter, about 30% of the mice which once showed paralysis developed hind limb paralysis again by 56 DPI. Histopathologically, the spinal cord lesion of paralysed mice was characterized by demyelination associated with infiltration of macrophages in the funiculus lateralis and by degeneration of neurons in the cornu ventrale. Virus antigens were detected in the cytoplasm of degenerated neurons and oligodendrocytes in the demyelinated lesions from 3 to 14 DPI. At 28 DPI, demyelinated lesions reduced in size due to prominent remyelination. At 56 DPI, infiltration of mononuclear cells mainly composed of anti-L3T4-positive (CD4+) T cells were observed in the cornu ventrale of the mice showing recurrence of hind limb paralysis. These results suggested that the early paralysis was mainly due to demyelination in funiculus lateralis caused by EMC-D and macrophages and that the late paralysis was due to degeneration of motor neurons, probably brought about by CD4+ T cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baek H. S., Yoon J. W. Role of macrophages in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J Virol. 1990 Dec;64(12):5708–5715. doi: 10.1128/jvi.64.12.5708-5715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E., Huber S. A., Sriram S. Animal models of picornavirus-induced autoimmune disease: their possible relevance to human disease. Lab Invest. 1990 Oct;63(4):432–446. [PubMed] [Google Scholar]
- Craighead J. E., McLane M. F. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science. 1968 Nov 22;162(3856):913–914. doi: 10.1126/science.162.3856.913. [DOI] [PubMed] [Google Scholar]
- Doi K., Matsuzaki H., Tsuda T., Onodera T. Rapid development of renal lesions in diabetic DBA mice infected with the D-variant of encephalomyocarditis virus (EMC-D). Br J Exp Pathol. 1989 Jun;70(3):275–281. [PMC free article] [PubMed] [Google Scholar]
- Helwig F. C., Schmidt C. H. A FILTER-PASSING AGENT PRODUCING INTERSTITIAL MYOCARDITIS IN ANTHROPOID APES AND SMALL ANIMALS. Science. 1945 Jul 13;102(2637):31–33. doi: 10.1126/science.102.2637.31. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Christianson W. T., Joo H. S. Pathogenic properties of encephalomyocarditis virus isolates in swine fetuses. Arch Virol. 1989;109(1-2):51–57. doi: 10.1007/BF01310517. [DOI] [PubMed] [Google Scholar]
- Lindsley M. D., Rodriguez M. Characterization of the inflammatory response in the central nervous system of mice susceptible or resistant to demyelination by Theiler's virus. J Immunol. 1989 Apr 15;142(8):2677–2682. [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURNANE T. G., CRAIGHEAD J. E., MONDRAGON H., SHELOKOV A. Fatal disease of swine due to encephalomyocarditis virus. Science. 1960 Feb 19;131(3399):498–499. doi: 10.1126/science.131.3399.498. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H., Doi K., Mitsuoka T., Tsuda T., Onodera T. Experimental encephalomyocarditis virus infection in Mongolian gerbils (Meriones unguiculatus). Vet Pathol. 1989 Jan;26(1):11–17. doi: 10.1177/030098588902600103. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J. L., Lampert P. W. Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann Neurol. 1983 Apr;13(4):426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Sriram S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988 May 1;140(9):2950–2955. [PubMed] [Google Scholar]
- Sriram S., Topham D. J., Huang S. K., Rodriguez M. Treatment of encephalomyocarditis virus-induced central nervous system demyelination with monoclonal anti-T-cell antibodies. J Virol. 1989 Oct;63(10):4242–4248. doi: 10.1128/jvi.63.10.4242-4248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M., Hirasawa K., Doi K. Lesions in the central nervous system of DBA/2 mice infected with the D variant of encephalomyocarditis virus (EMC-D). J Vet Med Sci. 1991 Dec;53(6):1013–1017. doi: 10.1292/jvms.53.1013. [DOI] [PubMed] [Google Scholar]
- Topham D. J., Adesina A., Shenoy M., Craighead J. E., Sriram S. Indirect role of T cells in development of polioencephalitis and encephalomyelitis induced by encephalomyocarditis virus. J Virol. 1991 Jun;65(6):3238–3245. doi: 10.1128/jvi.65.6.3238-3245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Zurbriggen A., Fujinami R. S. The relationship between viral RNA, myelin-specific mRNAs, and demyelination in central nervous system disease during Theiler's virus infection. Am J Pathol. 1990 Dec;137(6):1467–1479. [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W., McClintock P. R., Onodera T., Notkins A. L. Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J Exp Med. 1980 Oct 1;152(4):878–892. doi: 10.1084/jem.152.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W., Rodrigues M. M., Currier C., Notkins A. L. Long-term complications of virus-induced diabetes mellitus in mice. Nature. 1982 Apr 8;296(5857):566–569. doi: 10.1038/296566a0. [DOI] [PubMed] [Google Scholar]