Abstract
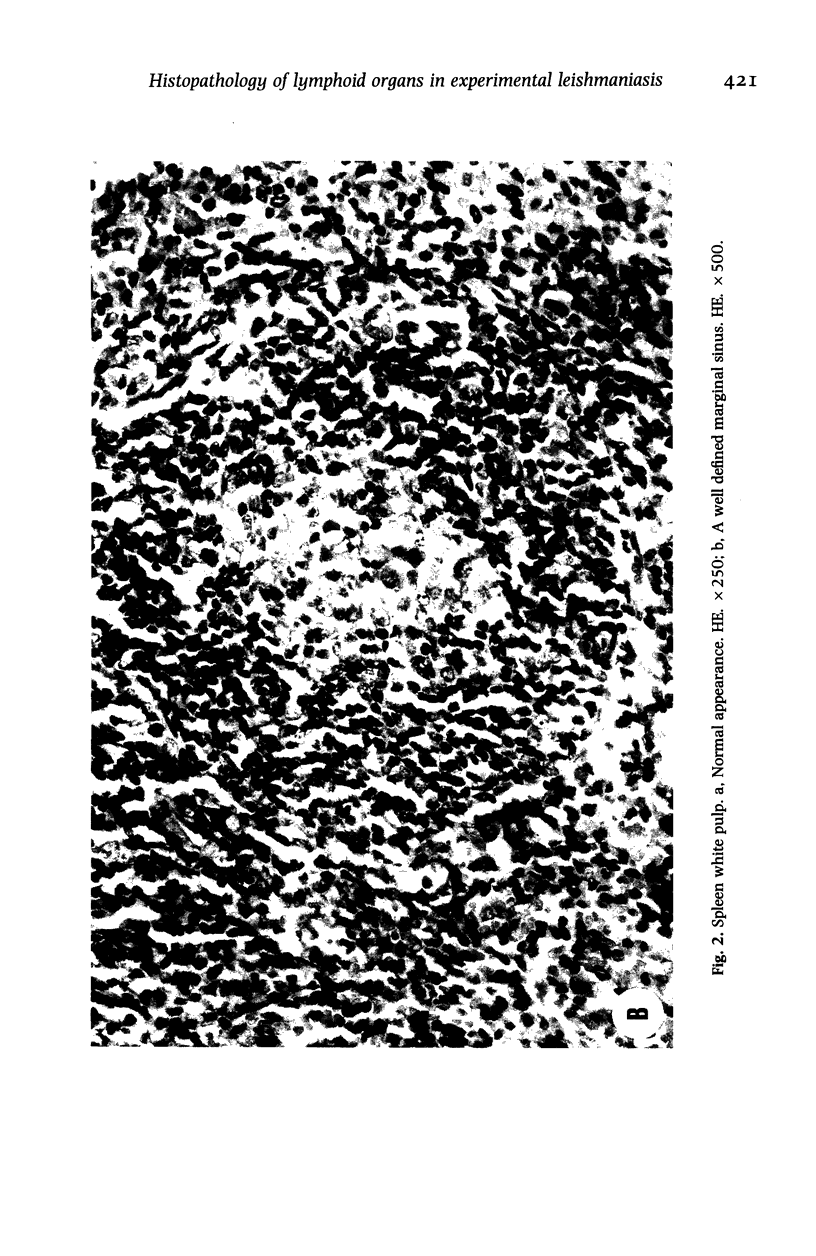
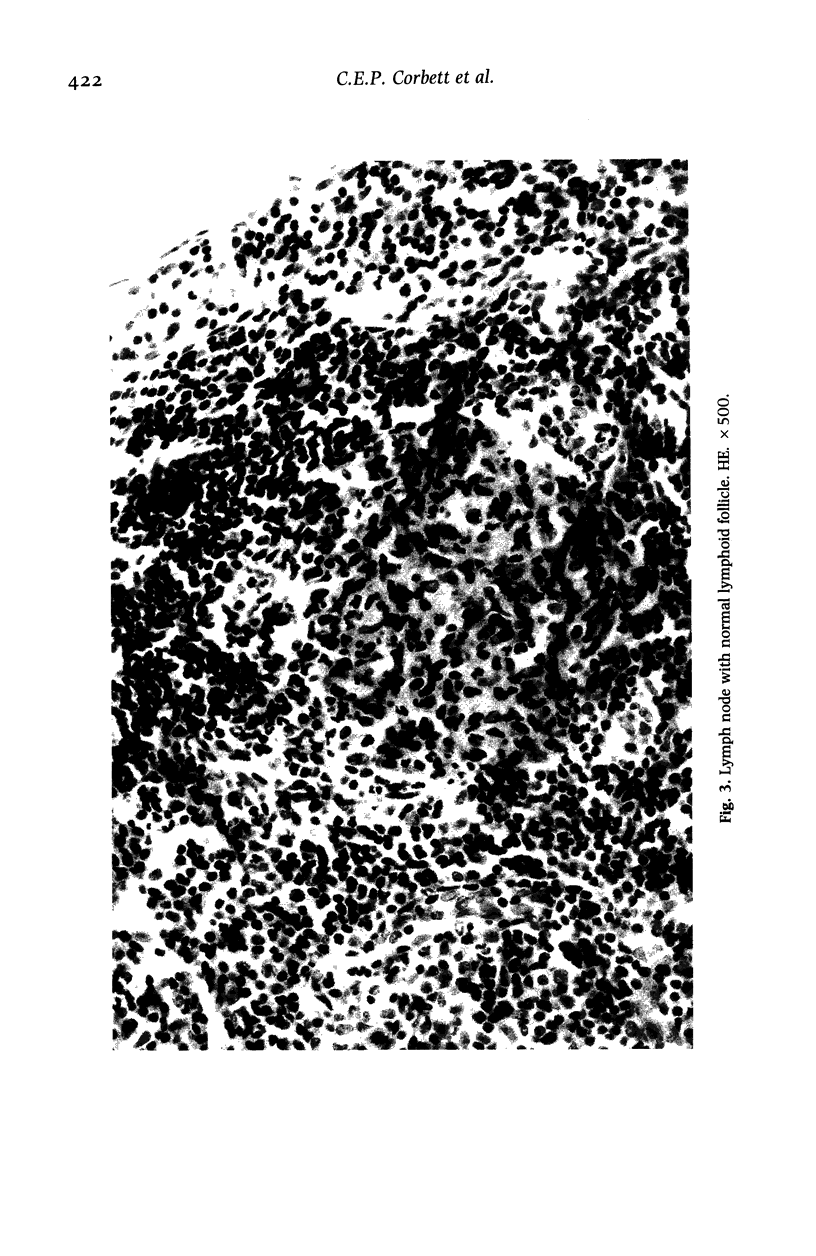
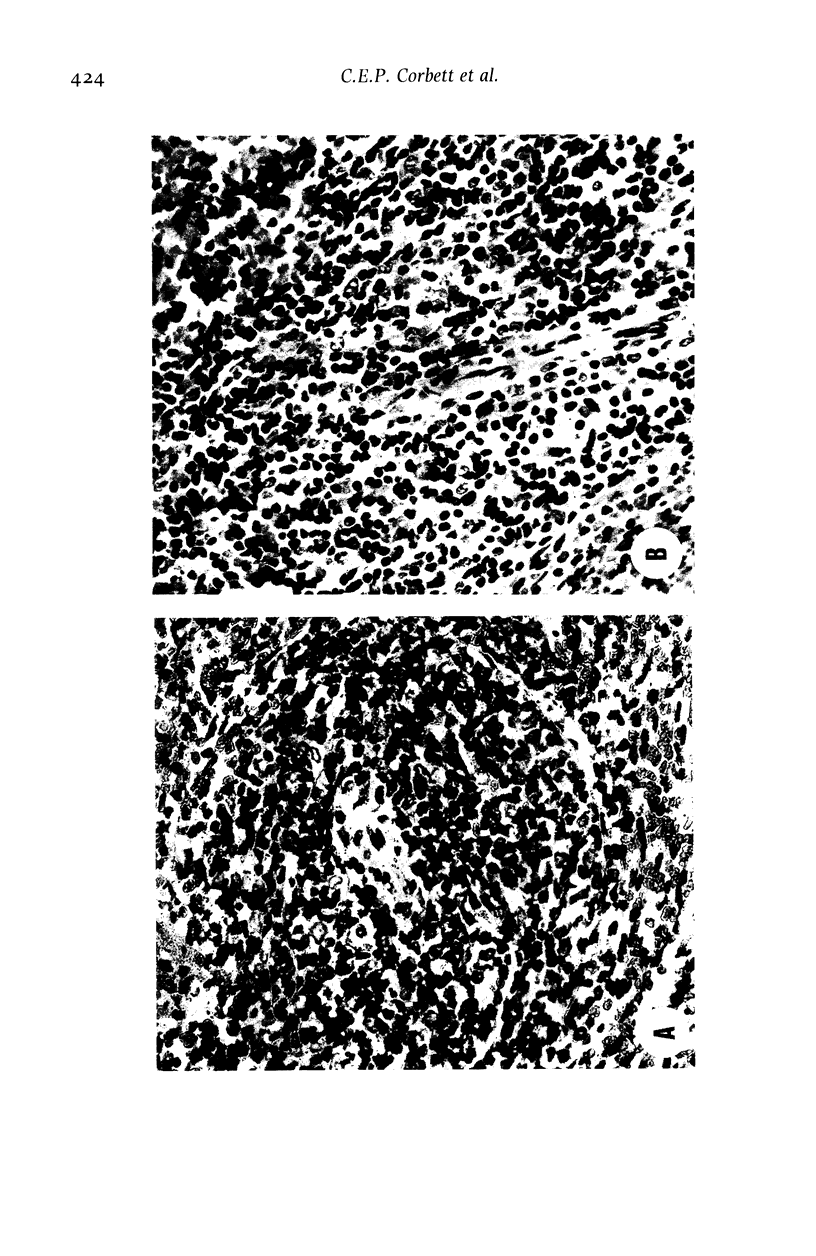
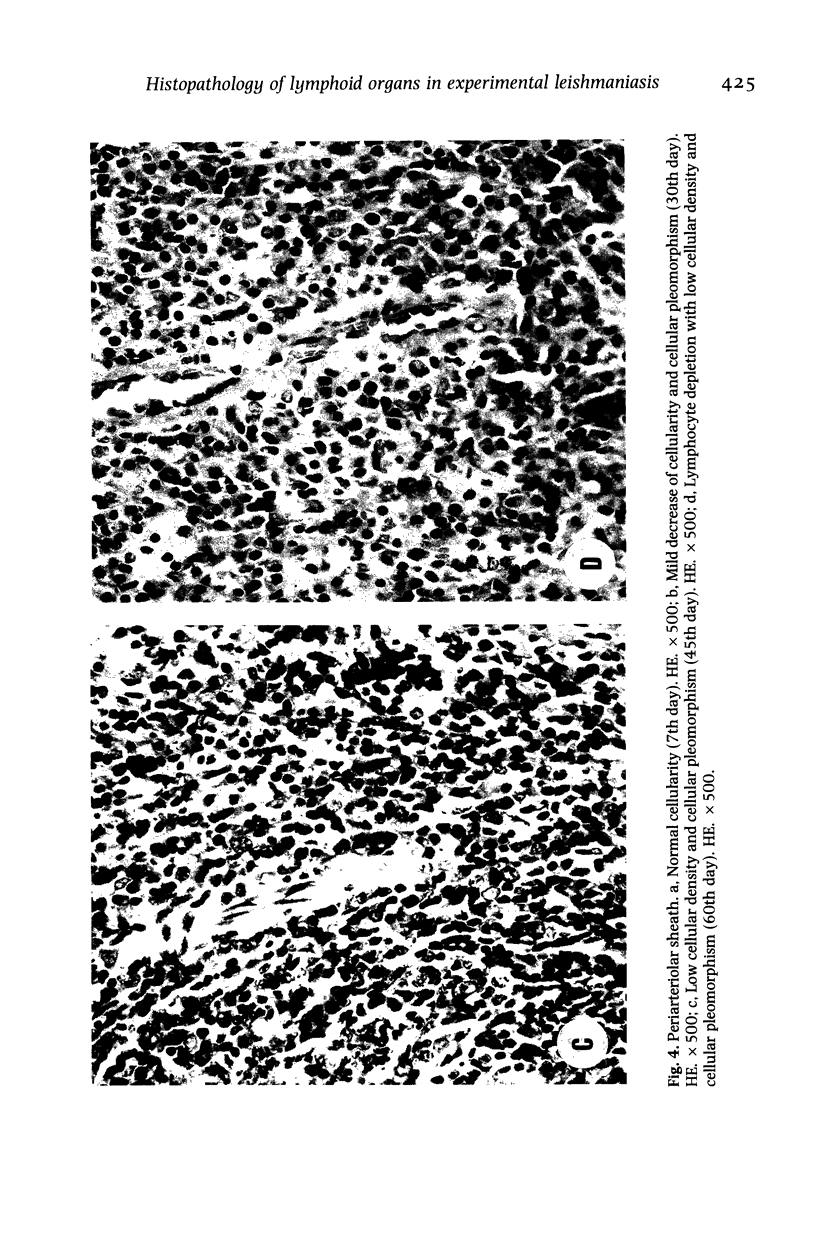
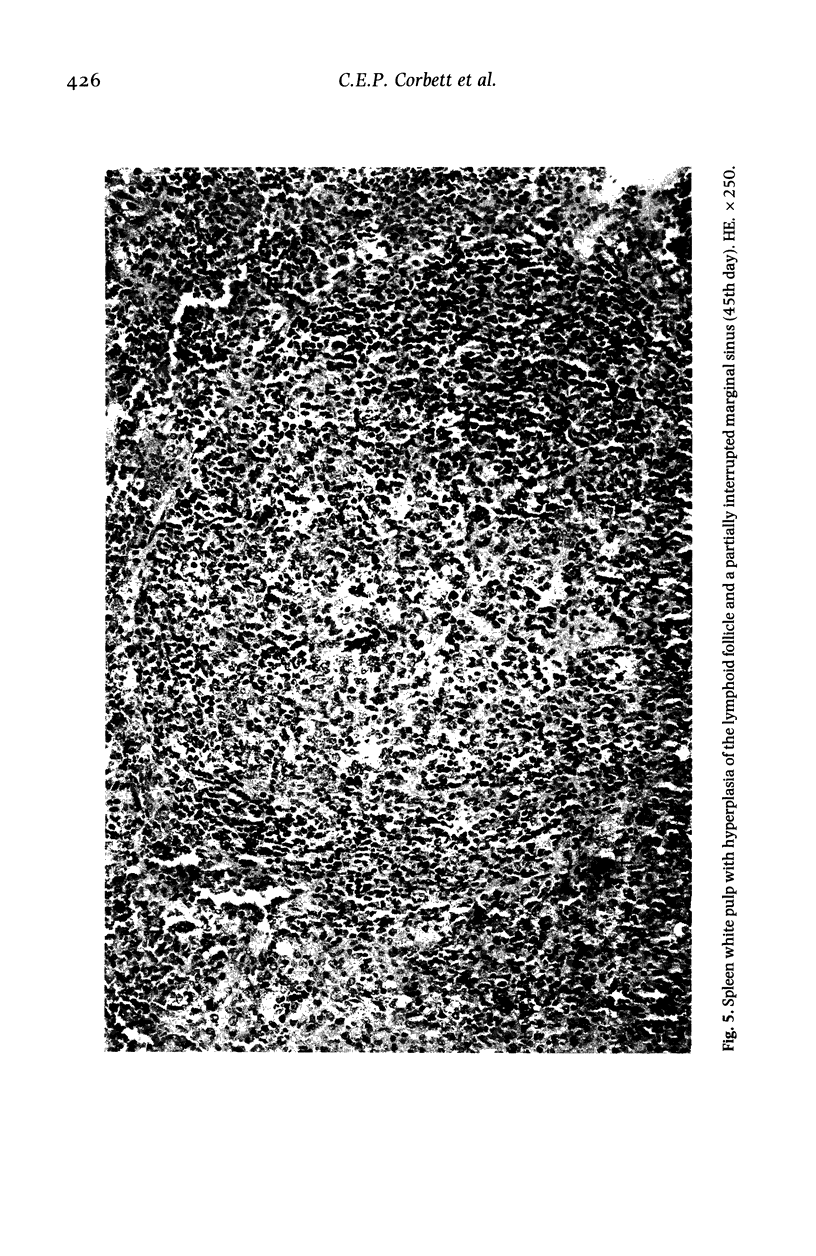
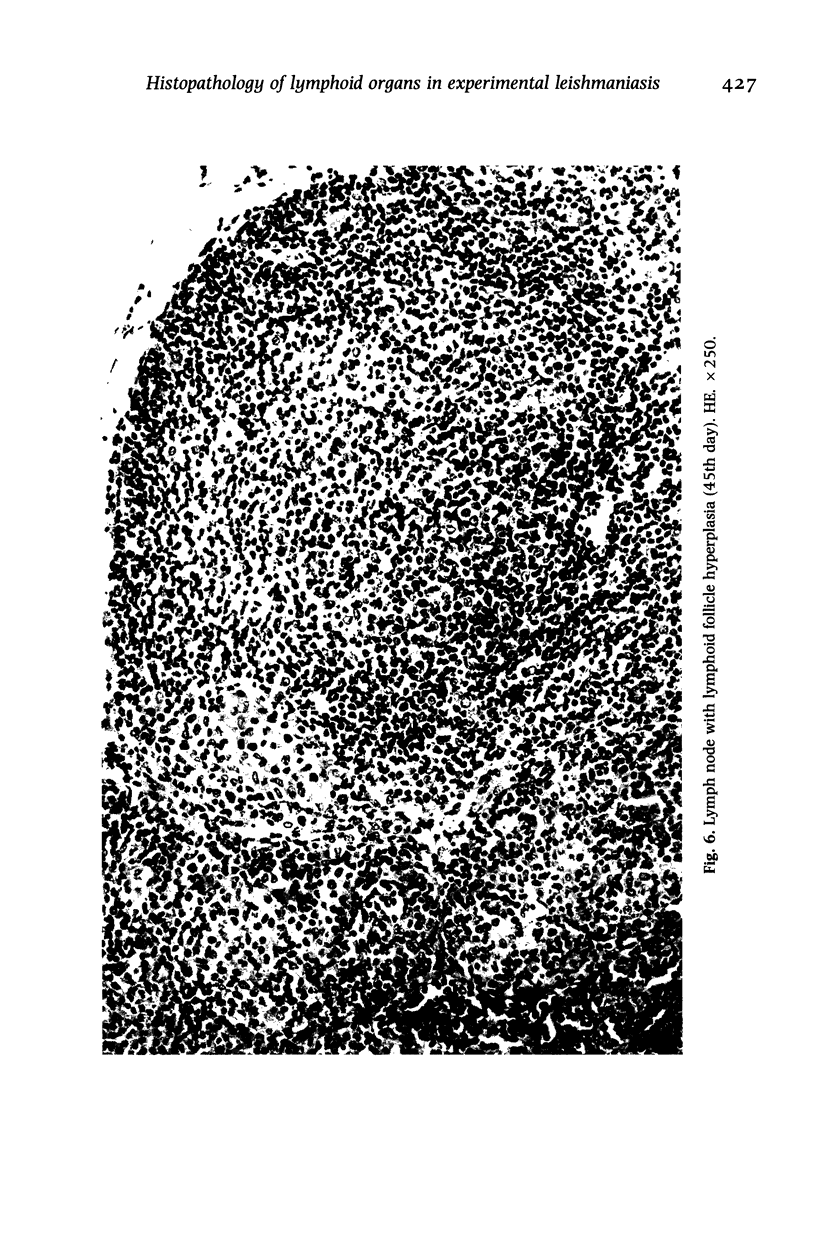
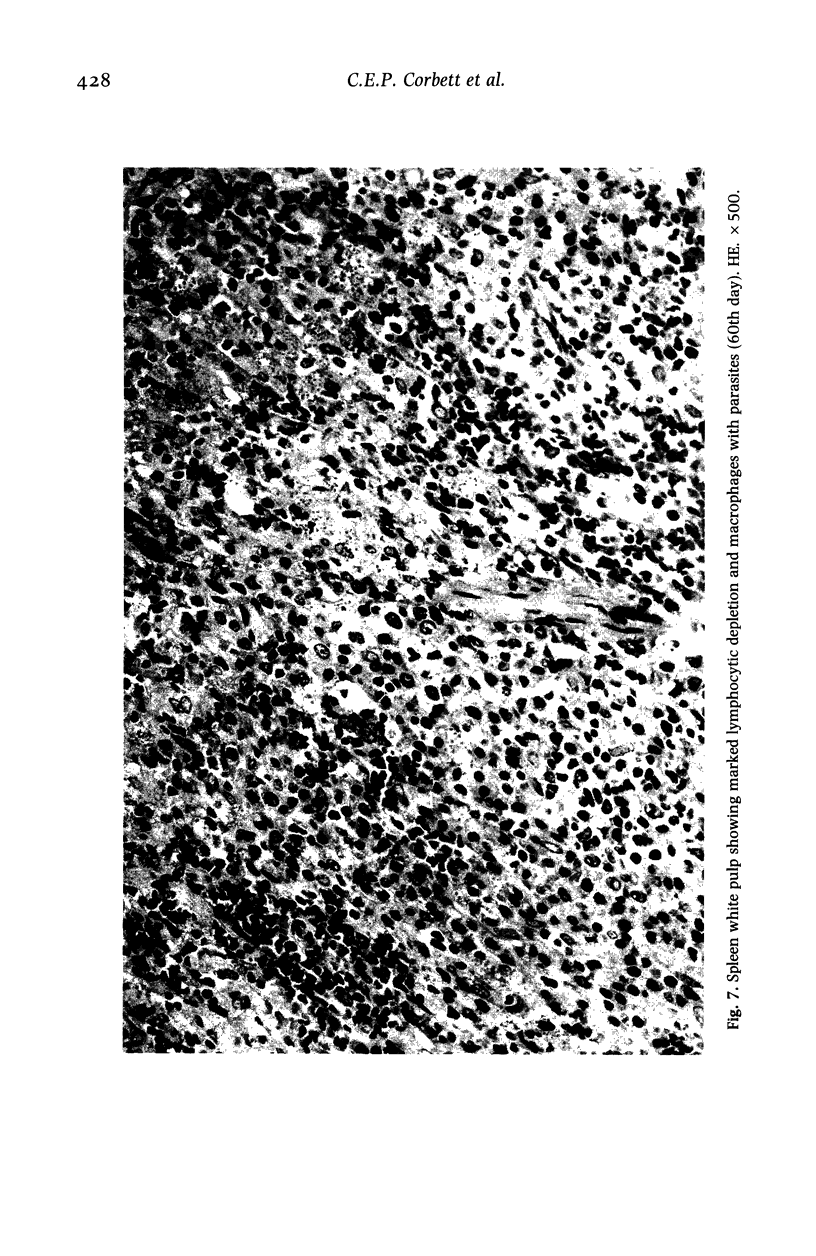
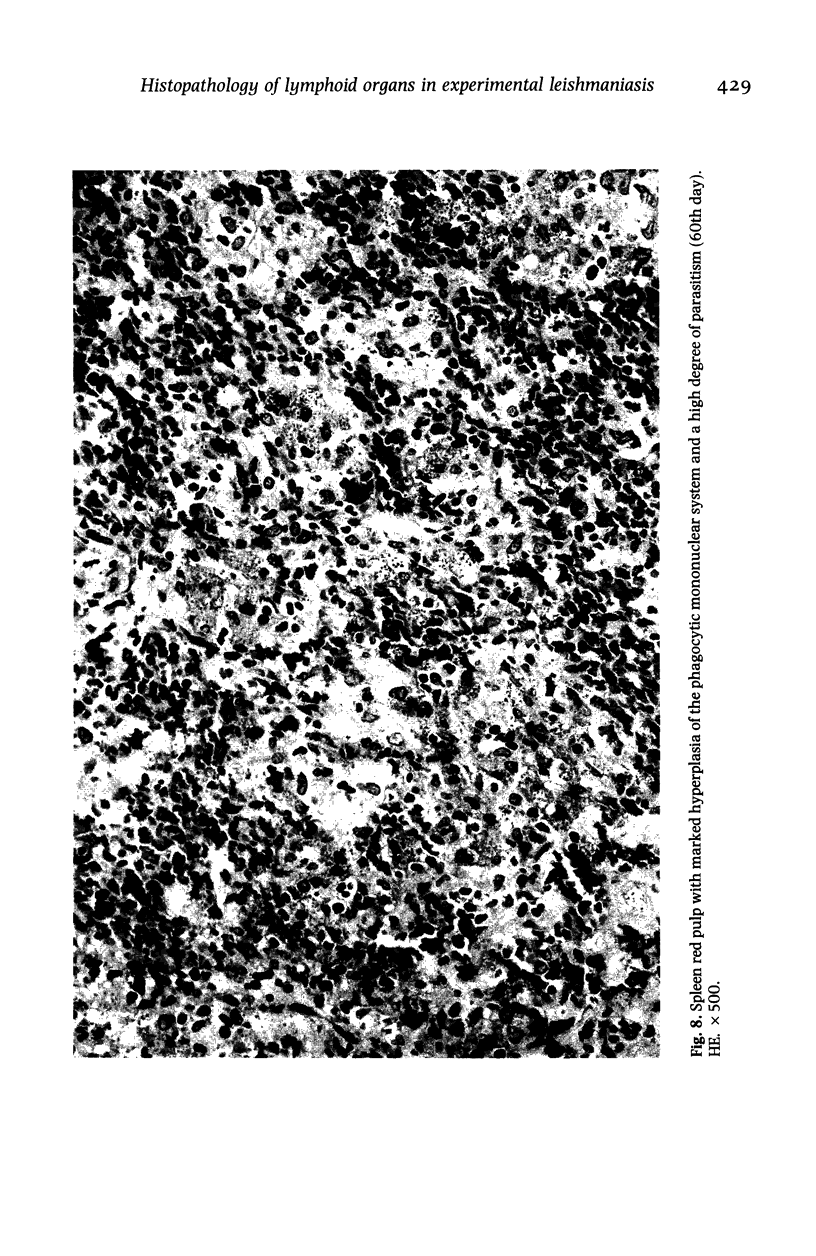
Hamsters (Mesocricetus auratus) were inoculated with L. (L.) chagasi and killed on days 7, 15, 30, 45 and 60 after infection. The lymphoid organs developed initial proliferation of the B lymphocyte zone with recovery by the 60th day group when pyroninophilic cells were prominent. The T lymphocyte area showed a progressive selective decrease of lymphocytes and cellular density with cellular pleomorphism including macrophages, plasma cells and reticular cells. The mean volume of the white pulp increased with the lymphoid follicle hyperplasia but returned to its initial level by day 60. The main red pulp change was marked hyperplasia of the phagocytic mononuclear cells containing parasites from the 30th day of infection onward. These changes are compatible with the humoral and cellular immunoresponse found in patients with visceral leishmaniasis (VL).
Full text
PDF

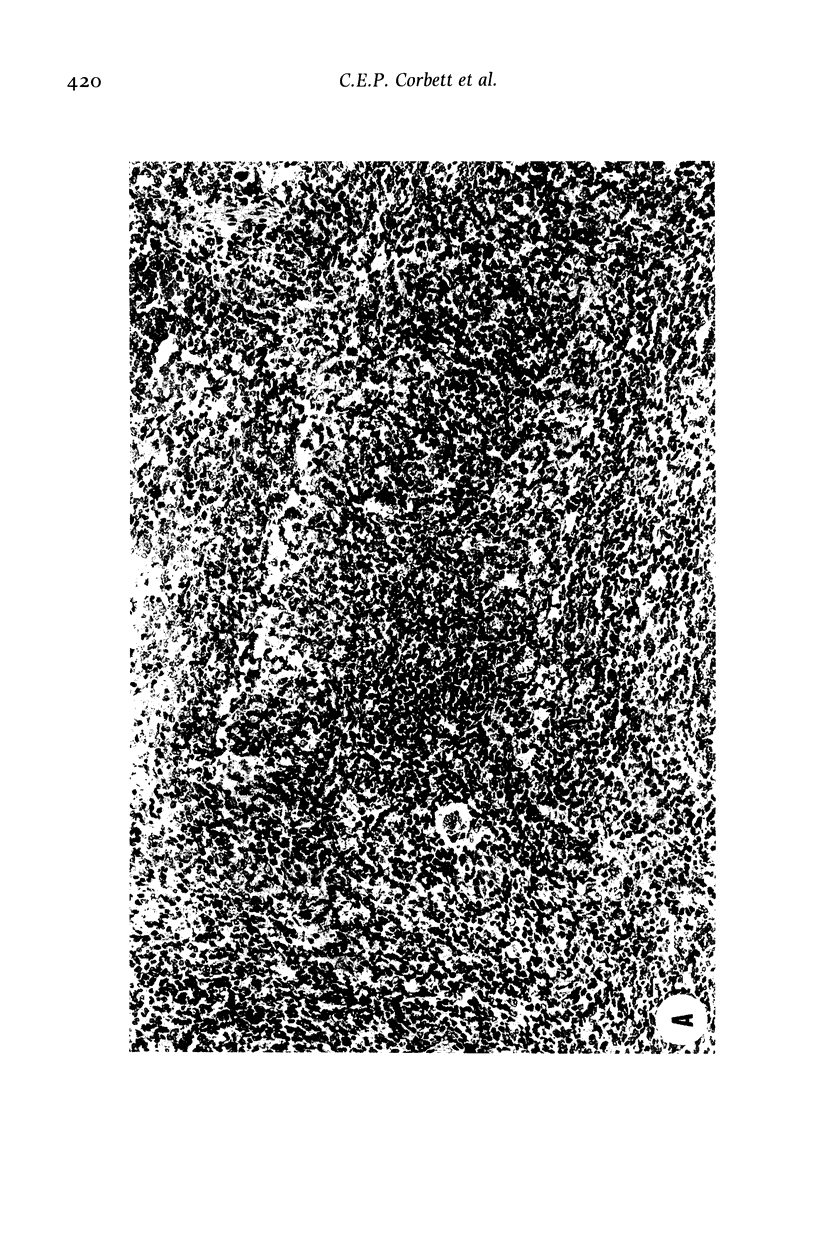




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikat B. K., Pathania A. G., Sehgal S., Bhattacharya P. K., Dutta U., Pasricha N., Singh S., Parmar R. S., Sahaya S., Prasad L. S. Immunological response in Indian kala-azar. Indian J Med Res. 1979 Oct;70:583–591. [PubMed] [Google Scholar]
- Andrade Z. A., Andrade S. G. Alguns novos aspectos da patologia do calazar. (Estudo morfológico de 13 casos necropsiados) Rev Inst Med Trop Sao Paulo. 1966 Nov-Dec;8(6):259–266. [PubMed] [Google Scholar]
- BELL D. W., CARMICHAEL J. A., WILLIAMS R. S., HOLMAN R. L., STEWART P. D. Localized leishmaniasis of lymph nodes; report of four cases. Br Med J. 1958 Mar 29;1(5073):740–743. doi: 10.1136/bmj.1.5073.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M. I., Corbett C. E. Histopathological and ultrastructural aspects of interstitial pneumonitis of experimental visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1984;78(5):683–688. doi: 10.1016/0035-9203(84)90242-6. [DOI] [PubMed] [Google Scholar]
- Gutierrez Y., Maksem J. A., Reiner N. E. Pathologic changes in murine leishmaniasis (Leishmania donovani) with special reference to the dynamics of granuloma formation in the liver. Am J Pathol. 1984 Feb;114(2):222–230. [PMC free article] [PubMed] [Google Scholar]
- Jehan J., Borel B., Chasles J., Herlin P., Leporrier M., Rousselot P. La leishmaniose viscérale. Etude anatomopathologique à propos d'une observation avec réaction épithélioïde. Arch Anat Cytol Pathol. 1982;30(4):251–254. [PubMed] [Google Scholar]
- Koech D. K., Iha D. W., Ho M., Wamachi A. N. Contribution of adherent cells and serum components to immune suppression in Kenyan visceral leishmaniasis. Am J Trop Med Hyg. 1987 May;36(3):501–504. doi: 10.4269/ajtmh.1987.36.501. [DOI] [PubMed] [Google Scholar]
- Meleney H. E. The Histopathology of Kala-Azar in the Hamster, Monkey, and Man. Am J Pathol. 1925 Mar;1(2):147–168.11. [PMC free article] [PubMed] [Google Scholar]
- Musumeci S., Schilirò G., Li Volti S., Sciotto A. Lymphocyte changes in Mediterranean kala-azar. Trans R Soc Trop Med Hyg. 1981;75(2):304–305. doi: 10.1016/0035-9203(81)90342-4. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis P., Keuning F. J. Germinal centres and the origin of the B-cell system. II. Germinal centres in the rabbit spleen and popliteal lymph nodes. Immunology. 1974 Mar;26(3):509–519. [PMC free article] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees P. H., Kager P. A., Muriithi M. R., Wambua P. P., Shah S. D., Butterworth A. E. Tuberculin sensitivity in kala-azar. Trans R Soc Trop Med Hyg. 1981;75(5):630–631. doi: 10.1016/0035-9203(81)90135-8. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Ardehali S. M., Amirhakimi G., Kharazmi A. Immunological features of kala-azar. Am J Trop Med Hyg. 1978 Nov;27(6):1079–1083. doi: 10.4269/ajtmh.1978.27.1079. [DOI] [PubMed] [Google Scholar]
- Scheinman J. I., Fish A. J. Human glomerular cells in culture. Three subcultured cell types bearing glomerular antigens. Am J Pathol. 1978 Jul;92(1):125–146. [PMC free article] [PubMed] [Google Scholar]
- Silveira N. P., Mendes N. F., Tolnai M. E. Tissue localization of two populations of human lymphocytes distinguished by membrane receptors. J Immunol. 1972 May;108(5):1456–1460. [PubMed] [Google Scholar]
- Veress B., Malik M. O., Satir A. A., el Hassan A. M. Morphological observations on visceral leishmaniasis in the Sudan. Trop Geogr Med. 1974 Jun;26(2):198–203. [PubMed] [Google Scholar]
- van Krieken J. H., von Schilling C., Kluin P. M., Lennert K. Splenic marginal zone lymphocytes and related cells in the lymph node: a morphologic and immunohistochemical study. Hum Pathol. 1989 Apr;20(4):320–325. doi: 10.1016/0046-8177(89)90040-3. [DOI] [PubMed] [Google Scholar]