Fig 10.
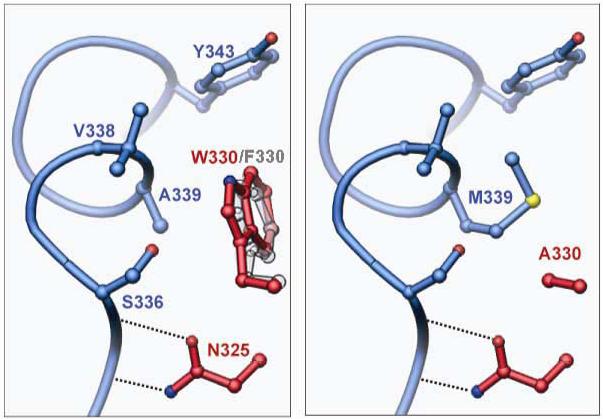
Second site suppression of W330A by A339M. Helix M from the ‘donor’ protomer is shown in blue, and two residues from the ‘recipient’ protomer in red. Left: In wild type Flp or Flp(W330F), Trp-330 or Phe-330 packs against the first turn of helix M, making van der Waals contacts with Ser-336, Val-338 and Ala-339 (see also Fig. 8). Right: Similar view of a model structure for the suppressor variant Flp(W330A, A339M). The program O 40 was used to replace the side chains at 330 and 339, and to find the rotamer of Met-339 that best fills the space vacated by the W330A mutation without causing steric conflicts with other residues.
